| CPC C07C 317/32 (2013.01) [A61K 31/10 (2013.01); A61K 31/277 (2013.01); A61K 31/343 (2013.01); A61K 31/353 (2013.01); A61K 31/365 (2013.01); A61K 31/381 (2013.01); A61K 31/404 (2013.01); A61K 31/415 (2013.01); A61K 31/416 (2013.01); A61K 31/4174 (2013.01); A61K 31/4192 (2013.01); A61K 31/4196 (2013.01); A61K 31/421 (2013.01); A61K 31/423 (2013.01); A61K 31/426 (2013.01); A61K 31/428 (2013.01); A61K 31/437 (2013.01); A61K 31/44 (2013.01); A61K 31/4412 (2013.01); A61K 31/47 (2013.01); A61K 31/472 (2013.01); A61K 31/4709 (2013.01); A61K 31/4965 (2013.01); A61K 31/50 (2013.01); A61K 31/505 (2013.01); A61K 31/5025 (2013.01); A61K 31/519 (2013.01); A61K 31/538 (2013.01); A61K 39/3955 (2013.01); A61K 45/06 (2013.01); C07C 255/53 (2013.01); C07C 317/22 (2013.01); C07C 317/36 (2013.01); C07D 209/34 (2013.01); C07D 211/86 (2013.01); C07D 213/57 (2013.01); C07D 213/71 (2013.01); C07D 213/73 (2013.01); C07D 213/74 (2013.01); C07D 213/84 (2013.01); C07D 215/48 (2013.01); C07D 217/04 (2013.01); C07D 231/12 (2013.01); C07D 231/56 (2013.01); C07D 233/64 (2013.01); C07D 237/20 (2013.01); C07D 239/42 (2013.01); C07D 241/12 (2013.01); C07D 249/06 (2013.01); C07D 249/08 (2013.01); C07D 263/32 (2013.01); C07D 263/57 (2013.01); C07D 265/36 (2013.01); C07D 277/30 (2013.01); C07D 277/56 (2013.01); C07D 277/64 (2013.01); C07D 307/83 (2013.01); C07D 311/22 (2013.01); C07D 333/64 (2013.01); C07D 401/04 (2013.01); C07D 401/10 (2013.01); C07D 409/10 (2013.01); C07D 413/10 (2013.01); C07D 471/04 (2013.01); C07D 487/04 (2013.01); C07C 2602/08 (2017.05); C07C 2602/10 (2017.05)] | 26 Claims |
|
1. A method of treating a cancer, said method comprising administering an effective amount of a compound, or a pharmaceutically acceptable salt, hydrate, or solvate thereof, to a subject in need thereof,
wherein said cancer is a cancer of the prostate, colon, rectum, pancreas, cervix, stomach, endometrium, uterus, brain, liver, bladder, ovary, testis, head, neck, skin, mesothelial lining, white blood cell, esophagus, breast, muscle, connective tissue, intestine, lung, adrenal gland, thyroid, kidney, or bone; or is glioblastoma, mesothelioma, renal cell carcinoma, gastric carcinoma, sarcoma, choriocarcinoma, cutaneous basocellular carcinoma, or testicular seminoma; or is melanoma, colorectal cancer, leukemia, lymphoma, Kaposi's sarcoma, or urothelieal carcinoma;
wherein the compound has a structure of Formula (II):
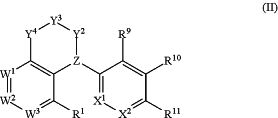 wherein
Z is N or CR6;
Y2, Y3, and Y4 are each independently selected from the group consisting of CR2R3, NR4, O, SO2, and a bond; and no more than one of Y2, Y3 and Y4 is a bond;
W1, W2, and W3 are each independently selected from the group consisting of CR5 and N;
R1 is selected from the group consisting of H, halogen, hydroxy, CN, NO2, —NRaRb, C1-4 alkyl, C1-4 haloalkyl, C1-4 alkoxy, C1-4 haloalkoxy, C1-4 hydroxyalkyl, C1-4 alkoxyC1-4 alkyl, C3-8 cycloalkyl, —S(O)2Ra, —C(O)NRaRb, —S(O)(═NH)Ra, and —S(O)2NRaRb;
each R2 and R3 are each independently selected from the group consisting of H, halogen, CN, NO2, OH, C1-6 alkyl, C1-6 haloalkyl, C1-6 alkoxy, C1-6 haloalkoxy, C1-6 hydroxyalkyl, C1-4 alkoxyC1-4alkyl, C3-8 cycloalkyl, —S(O)2Ra, —CO2Ra, —C(O)Ra, —C(O)NRaRb, —S(O)2NRaRb, —S(O)(═NH)Ra, and —NRaRb;
each R4 is independently selected from H, C1-4 alkyl, C3-8 cycloalkyl, and —C(O)Ra,
each R5 is independently selected from the group consisting of H, halogen, CN, NO2, C1-6 alkyl, C1-6 haloalkyl, C1-6 alkoxy, C1-6 haloalkoxy, C1-6 hydroxyalkyl, C1-4 alkoxyC1-4alkyl, C3-8 cycloalkyl, S(O)2Ra, —CO2Ra, —C(O)Ra, —C(O)NRaRb, —S(O)2NRaRb, —S(O)(═NH)Ra, and —NRaRb;
X1 is N or CR8a;
X2 is N or CR8b;
R6 is selected from the group consisting of H, C1-4 alkyl, OH, F, and CN;
R8a and R8b are independently selected from the group consisting of H, halogen, CN, NH2, NO2, C1-6 alkyl, C1-6 haloalkyl, C1-6 alkoxy, C1-6 haloalkoxy, C1-6 hydroxyalkyl, C1-4 alkoxyC1-4alkyl, C3-6 cycloalkyl, —C(O)NRaRb, —S(O)2NRaRb, and —S(O)2Ra;
R9 and R10 are independently selected from the group consisting of H, halogen, CN, NO2, C1-6 alkyl, C1-6 haloalkyl, C1-6 alkoxy, C1-6 haloalkoxy, C1-6 hydroxyalkyl, C1-6 hydroxyhaloalkyl, C1-4 alkoxyC1-4alkyl, C3-8 cycloalkyl, —C(O)Ra, —C(O)ORa, —C(O)NRaRb, —S(O)2NRaRb, and —S(O)2Ra;
R11 is selected from the group consisting of H, halogen, CN, NO2, C1-6 alkyl, C1-6 haloalkyl, C1-6 alkoxy, C1-6 haloalkoxy, C1-6 hydroxyalkyl, C1-6 hydroxyhaloalkyl, C1-4 alkoxyC1-4alkyl, C3-8 cycloalkyl, —NRcRb, —C(O)NRcRb, —C(O)OH, —S(O)2NRcRb, —S(O)(═NH)Rc, —S(O)2Rc, phenyl, 5- to 6-membered heterocyclic ring, and 5- to 10-membered heteroaryl ring, wherein the heterocyclic and heteroaryl rings have from 1-3 heteroatoms as ring vertices selected from N, O, and S; wherein the phenyl is optionally fused to a 5- or 6-membered heterocycle having from 1-2 heteroatoms as ring vertices selected from N, O, and S; and wherein the phenyl, heterocyclic or heteroaryl rings are optionally substituted with from one to three members independently selected from halogen, CN, NO2, NH2, C(O)NH2, S(O)2CH3, —CH2NH2, C1-6 alkyl, C1-6 haloalkyl, C1-6 alkoxy, C1-6 haloalkoxy, C1-6 hydroxyalkyl, C1-6 hydroxyhaloalkyl and C1-4 alkoxyC1-4alkyl; optionally wherein two members attached to the same carbon of the heterocyclic ring taken together form ═CH2 or oxo (═O) group;
or R9 and R10 are combined to form a 5-membered carbocyclic or heterocyclic ring or a 6-membered carbocyclic, heterocyclic or heteroaryl ring, which is optionally substituted with one or more substituents independently selected from R12, R13, R14, R15, R16, R17, R18, and R19, the heterocyclic or heteroaryl ring each have from 1-4 heteroatoms as ring vertices selected from N, O and S;
or R10 and R11 are combined to form a 5- or 6-membered carbocyclic, heterocyclic or heteroaryl ring, which is optionally substituted with one or more substituents independently selected from R12, R13, R14, R15, R16, R17, R18, and R19, the heterocyclic or heteroaryl ring each have from 1-4 heteroatoms as ring vertices selected from N, O and S;
each of R12, R13, R14, R15, R16, R17, R18, and R19 is independently selected from the group consisting of H, halogen, CN, OH, C1-4 alkyl, C1-4 haloalkyl, C1-4 alkoxy, C1-4 haloalkoxy, C1-4 hydroxyalkyl, C1-4 alkoxyC1-4 alkyl, and —NRaRb; or two R12, R13, R14, R15, R16, R17, R18, and R19 moieties on the same carbon atom combine to form an oxo group;
each Ra and Rb is independently selected from the group consisting of H, C1-8 alkyl, C1-8 alkoxy, C1-8 haloalkyl, C1-8 haloalkoxy, and C1-8 hydroxyalkyl; and
Rc, when present, is selected from the group consisting of H, C1-8 alkyl, C1-8 alkoxy, C1-8 haloalkyl, C1-8 haloalkoxy, C1-8 hydroxyalkyl, C3-6 cycloalkyl, 3- to 6-membered heterocycloalkyl, and 5- or 6-membered heteroaryl, wherein the heterocycloalkyl or heteroaryl ring each have from 1-4 heteroatoms as ring vertices selected from N, O and S.
|