| CPC A61K 31/52 (2013.01) [A61K 45/06 (2013.01); A61P 35/00 (2018.01)] | 30 Claims |
|
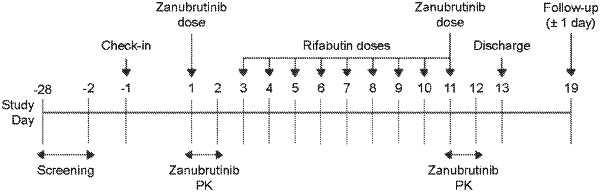
1. A method of treating or delaying progression of a B-cell proliferative disorder in a patient receiving a moderate CYP3A inducer, the method comprising
concomitantly administering to the patient zanubrutinib, or a pharmaceutically acceptable salt thereof, at a total daily dose of about 640 mg, and the moderate CYP3A inducer,
wherein the B-cell proliferative disorder is chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), Waldenström macroglobulinemia (WM), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), or follicular lymphoma (FL).
|