| CPC A61K 31/505 (2013.01) [A61K 9/209 (2013.01); A61K 9/2018 (2013.01); A61K 9/2813 (2013.01); A61K 31/397 (2013.01)] | 15 Claims |
|
1. A two-layer oral tablet comprising:
(a) rosuvastatin or its pharmaceutically acceptable salts, esters, hydrates or solvates;
(b) ezetimibe or its pharmaceutically acceptable salts, esters, hydrates or solvates;
wherein the tablet comprises one ezetimibe layer and one rosuvastatin layer;
wherein said ezetimibe layer comprises a granulate and an extragranulate phase;
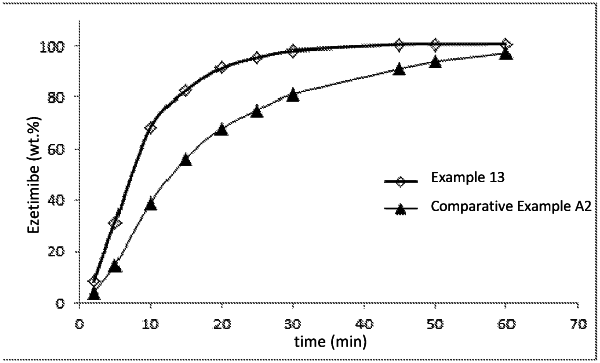
wherein the granulate comprises ezetimibe or its pharmaceutically acceptable salts, esters, hydrates or solvates;
wherein the extragranulate phase comprises stearic acid or its acceptable salts as a glidant at a concentration of 0.15 to 0.5% by weight, including the limit values, relative to the weight of the ezetimibe layer, and at least one further pharmaceutically acceptable excipient comprising a filler, a binder, or a disintegrant; and
wherein said rosuvastatin layer consists of rosuvastatin or its pharmaceutically acceptable salt, ester, hydrate or solvate, and at least one pharmaceutically acceptable excipient, wherein said pharmaceutically acceptable excipient(s) are non-basic, and
wherein the rosuvastatin layer does not contain a basic excipient.
|