| CPC A61K 31/497 (2013.01) [A61K 31/4155 (2013.01); A61K 31/454 (2013.01); A61K 31/5377 (2013.01); A61K 31/551 (2013.01); A61K 45/06 (2013.01); C07D 401/10 (2013.01); C07D 401/14 (2013.01); C07D 403/12 (2013.01); C07D 403/14 (2013.01); C07D 405/14 (2013.01); C07D 413/14 (2013.01)] | 19 Claims |
|
1. A method for the treatment of cancer, which method comprises administering to a patient a compound of the formula (0):
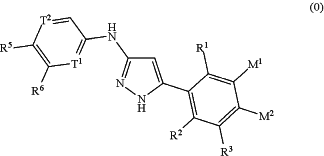 or a salt, N-oxide or tautomer thereof, wherein:
T1 and T2 are N;
R1 is selected from hydrogen, fluorine, C1-4 hydrocarbyl and C1-4 hydrocarbyloxy;
R2 is selected from hydrogen, fluorine, C1-4 hydrocarbyl and C1-4 hydrocarbyloxy;
R3 is selected from hydrogen, methyl, fluorine, chlorine and bromine;
one of M1 and M2 is a group R4 selected from hydrogen, methyl, fluorine, chlorine and bromine; and the other of M1 and M2 is a moiety —A—R7;
R5 is selected from hydrogen, cyano, C1-3 alkyl, cyclopropyl, chlorine, carboxy, and C1-3-alkoxy-carbonyl;
R6 is selected from hydrogen, fluorine, C1-4 alkyl; and C1-4 alkoxy optionally substituted with NRdRe where Rd and Re are the same or different and each is selected from hydrogen and C1-4 alkyl, or NRdRe forms a 4 to 7 membered saturated heterocyclic ring optionally containing a second heteroatom ring member selected from N, O and S and oxidized forms of S, the saturated heterocyclic ring being optionally substituted with one or more substituents selected from oxo, methyl, hydroxy and fluorine;
A is selected from:
(i) a bond;
(ii) (CRpRq)x where Rp and Rq are each independently hydrogen or methyl and x is 1 to 4;
(iii) an oxygen atom;
(iv) a group NRr wherein Rr is hydrogen or methyl; and
(v) a saturated chain of 2 to 10 chain members in length containing at least one carbon atom chain member, at least one heteroatom chain member selected from nitrogen and oxygen, and optionally one or more further carbon atom chain members and/or heteroatom chain members selected from nitrogen, oxygen, sulphur, sulphinyl and sulphonyl; the saturated chain being optionally substituted with one or more substituents selected from ═O, C1-4 hydrocarbyl, fluoro-C1-4 hydrocarbyl, hydroxy-C1-4 hydrocarbyl, C1-2-alkoxy-C1-4 hydrocarbyl, and fluorine wherein two hydrocarbyl substituents on the same carbon atom may optionally link to form a ring of three to five ring members;
R7 is a group Cyc1 wherein Cyc1 is a carbocyclic or heterocyclic non-aromatic group of 3 to 10 ring members of which 0 to 3 are selected from O, N and S and oxidised forms thereof, the carbocyclic or heterocyclic non-aromatic group being optionally substituted with one or more substituents R8;
R8 is selected from:
halogen;
oxo;
cyano;
nitro;
a carbocyclic or heterocyclic group having from 3 to 12 ring members, of which 0, 1, 2, 3 or 4 are heteroatom ring members selected from O, N and S and oxidised forms thereof, the carbocyclic or heterocyclic group being optionally substituted with one or more substituents R9; and
a group Ra—Rb;
Ra is a bond, O, CO, X1C(X2), C(X2)X1, X1C(X2)X1, S, SO, SO2, NRc, SO2NRc or NRcSO2;
Rb is selected from:
hydrogen;
a carbocyclic and heterocyclic group having from 3 to 12 ring members, of which 0, 1, 2, 3 or 4 are heteroatom ring members selected from O, N and S and oxidised forms thereof, the carbocyclic or heterocyclic group being optionally substituted with one or more substituents R9;
an acyclic C1-12 hydrocarbyl group optionally substituted with one or more substituents selected from hydroxy; oxo; halogen; cyano; nitro; carboxy; amino; mono- or di-C1-6 non-aromatic hydrocarbylamino; and carbocyclic and heterocyclic groups having from 3 to 12 ring members, of which 0, 1, 2, 3 or 4 are heteroatom ring members selected from O, N and S and oxidised forms thereof, the carbocyclic or heterocyclic group being optionally substituted with one or more substituents R9; wherein one or more but not all of the carbon atoms of the acyclic C1-12 hydrocarbyl group may optionally be replaced by O, S, SO, SO2, NRc, X1C(X2), C(X2)X1 or X1C(X2)X1;
Rc is selected from:
hydrogen;
a carbocyclic and heterocyclic group having from 3 to 12 ring members, of which 0, 1, 2, 3 or 4 are heteroatom ring members selected from O, N and S and oxidised forms thereof, the carbocyclic or heterocyclic group being optionally substituted with one or more substituents R9;
an acyclic C1-12 hydrocarbyl group optionally substituted with one or more substituents selected from hydroxy; oxo; halogen; cyano; nitro; carboxy; amino; mono- or di-C1-8 non-aromatic hydrocarbylamino; and carbocyclic and heterocyclic groups having from 3 to 12 ring members, of which 0, 1, 2, 3 or 4 are heteroatom ring members selected from O, N and S and oxidised forms thereof, the carbocyclic or heterocyclic group being optionally substituted with one or more substituents R9; wherein one or more but not all of the carbon atoms of the acyclic C1-12 hydrocarbyl group may optionally be replaced by O, S, SO, SO2, NH, N-C1-4 alkyl, C(O)O, OC(O), NH(CO), C(O)NH, NH(CO)NH, N(C1-4 alkyl)C(O), C(O)N(C1-4 alkyl)
X1 is O, S or NRc; and
X2 is ═O, ═S or ═NRc;
wherein R9 is selected from R8 provided that when the substituents R9 contain a carbocyclic or heterocyclic group, the said carbocyclic or heterocyclic group is unsubstituted or substituted with one or more substituents R10;
R10 is selected from halogen, oxo, cyano, and an acyclic C1-6 hydrocarbyl group optionally substituted with one or more substituents selected from hydroxy; oxo; halogen; cyano; carboxy; amino; mono- or di-C1-2 alkylamino; wherein one but not all of the carbon atoms of the acyclic C1-6 hydrocarbyl group may optionally be replaced by O, S, SO, SO2, NH or NMe;
R11 is selected from amino, Hyd1, NH-Hyd1, N(Hyd1)2; and Cyc1;
Hyd1 is a non-aromatic C1-6 hydrocarbyl group optionally substituted by one or more substituents selected from halogen, cyano, hydroxy, amino and Cyc1, wherein one or two of the carbon atoms of the non-aromatic C1-6 hydrocarbyl group may optionally be replaced by O, NH, N-Hyd2, C(═O), S, SO or SO2, provided that at least one carbon atom of the hydrocarbyl group remains;
Hyd2 is a C3-4 hydrocarbyl group;
and wherein in any group consisting of or containing a hydrocarbyl moiety, the hydrocarbyl moiety is a hydrocarbon group optionally containing one or more single, double or triple carbon-carbon bonds or combinations thereof;
wherein the cancer is selected from:
carcinomas of the bladder, breast, colon, kidney, epidermis, liver, lung, oesophagus, ovary, pancreas, stomach, cervix, thyroid, prostate, gastrointestinal system, or skin;
hematopoieitic tumours;
tumours of mesenchymal origin;
leukaemia and lymphomas;
tumours of the central or peripheral nervous system;
melanoma; and
fibrosarcoma, rhabdomyosarcoma, osteosarcoma or Ewing's sarcoma.
|