| CPC A61K 31/22 (2013.01) [A61P 9/06 (2018.01); A61P 9/10 (2018.01); A61P 9/12 (2018.01); A61P 25/02 (2018.01); A61P 25/06 (2018.01); C07C 229/34 (2013.01); A61K 31/4178 (2013.01); A61K 31/423 (2013.01); A61K 2300/00 (2013.01); C07B 2200/13 (2013.01)] | 12 Claims |
|
1. A crystalline form of a calcium salt of N-(3-carboxyl-1-oxopropyl)-(4S)-(p-phenylphenylmethyl) -4-amino-(2R)-methyl butanoic acid ethyl ester having the formula (I)
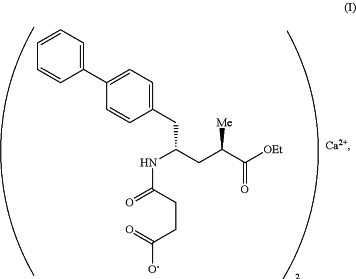 wherein the crystalline form is Modification B or hydrate form HB, wherein
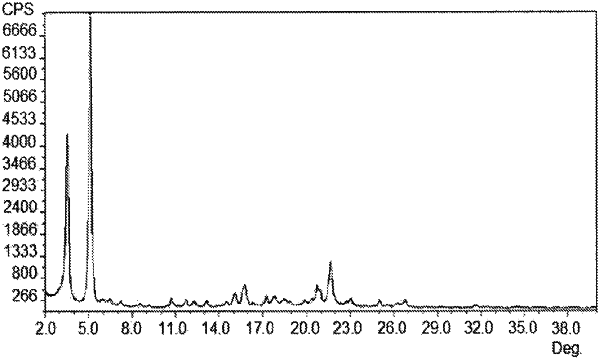
Modification B is characterized in that said form has an X-ray powder diffraction pattern comprising 2θ values (±0.1°) (CuKα; 45 kV, 40 mA;λ=1.540598 Å) selected from 4.6, 6.0, 12.6, 15.5, 16.6, 19.6 and 22.5 degrees at a temperature of 20 to 25° C., and
Modification HB is characterized in that said form has an X-ray powder diffraction pattern comprising 2θ values (±0.1°) (CuKα; 45 kV, 40 mA;λ=1.540598 Å) selected from 3.6, 6.4, 8.4, 14.6, 15.3, 16.8, 17.8, 19.7 and 20.6 degrees at a temperature of 20 to 25° C.
|