| CPC A61K 31/05 (2013.01) [A61K 8/33 (2013.01); A61K 8/347 (2013.01); A61K 8/602 (2013.01); A61K 8/676 (2013.01); A61K 8/70 (2013.01); A61K 8/733 (2013.01); A61K 8/738 (2013.01); A61K 9/0019 (2013.01); A61K 9/06 (2013.01); A61K 9/08 (2013.01); A61K 9/19 (2013.01); A61K 31/03 (2013.01); A61K 31/085 (2013.01); A61K 31/09 (2013.01); A61K 31/167 (2013.01); A61K 31/375 (2013.01); A61K 31/655 (2013.01); A61K 31/7034 (2013.01); A61K 45/06 (2013.01); A61K 47/36 (2013.01); A61K 47/40 (2013.01); A61Q 7/00 (2013.01); A61Q 19/08 (2013.01); Y02A 50/30 (2018.01)] | 23 Claims |
|
1. A composition comprising a first autophagy modulating compound dispersed in a hydrogel, wherein the first autophagy modulating compound has the structure (I):
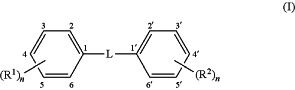 wherein L is a linker comprising —C≡C—;
R1 and R2 are independently substituents at any available position of the phenyl rings;
m and n are, independently, 0, 1, 2, or 3, representing the number of substituents on the rings, respectively, and at least one of m or n must be ≥1;
wherein each of R1 and R2 is independently selected from:
R5, wherein R5 is selected from (C1-C6)alkyl, (C2-C6)alkenyl, or (C2-C6)alkynyl, optionally substituted with 1 to 3 substituents selected from —OH, —SH, -halo, —NH2, or NO2;
YR6, wherein Y is O, S, or NH; and R6 is selected from H or R5;
ZR5, wherein Z is —N(C═O)— or —O(C═O)—;
halo;
NO2;
SO3Na;
azide; and
glycosides;
and salts thereof;
with the proviso that the first autophagy modulating compound is not resveratrol or 4,4′-(ethyne-1,2-diyl)diphenol (TOLECINE, also known as 4,4′-dihydroxytolan).
|