| CPC A61F 2/856 (2013.01) [A61F 2/07 (2013.01); A61F 2/2418 (2013.01); A61F 2/82 (2013.01); A61F 2/962 (2013.01)] |
| AS A RESULT OF REEXAMINATION, IT HAS BEEN DETERMINED THAT: |
| Claims 8 and 14-17 are cancelled. |
| Claim 1 is determined to be patentable as amended. |
| Claims 2-7 and 9-13, dependent on an amended claim, are determined to be patentable. |
| New claims 18 and 19 are added and determined to be patentable. |
|
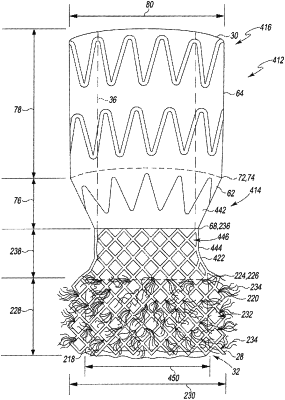
1. A transcatheter heart valve assembly comprising:
an outer frame, wherein the outer frame is formed from a metallic material and defines:
a first open cell portion [ , the first open cell portion defining at least two rows of cells formed from the metallic material] ; and
a second open cell portion extending from and continuous with the first open cell portion and defining a construction that differs from the construction of the first open cell portion;
an inner frame that houses a prosthetic heart valve, wherein the inner frame is a graft covering extending around at least a portion of the prosthetic heart valve for providing sealing to the heart valve, wherein the graft covering covers the first open cell portion but does not extend to the second open cell portion,
wherein the outer frame is secured to the graft covering by a plurality of stitches,
a sealing material positioned externally to the outer frame for providing sealing between the outer frame and a patient's anatomical wall to prevent paravalvular leaks, wherein the sealing material includes a plurality of outwardly extending fibers that extend outwardly of the outer frame to form the sealing material, wherein the sealing material is attached to the first open cell portion of the outer frame and extends over [ the at least two rows of cells in the ] at least a portion of the first open cell portion,
wherein the valve assembly is balloon expandable and expansion of the valve assembly presses the sealing material against native leaflets of the aorta of the patient [ to compress the fibers against the native leaflets to form the seal] ,
wherein the valve assembly has a radially compressed orientation and a radially expanded orientation [ ,
wherein the sealing material is positioned against the outer frame in the radially compressed orientation and the radially expanded orientation,
wherein the valve assembly is sized and shaped to be delivered endovascularly through a femoral artery of the patient,
wherein the sealing material defines a fiber density such that portions of the at least two rows of cells of the outer frame behind the sealing material are visible before the heart valve assembly is placed in the radially compressed orientation] .
|
|
[ 18. The valve assembly of claim 1, wherein the second open cell portion tapers inwardly relative to the first open cell portion.]
|
|
[ 19. The valve assembly of claim 1, wherein the graft covering is low-profile polyester, ePTFE, or other nonporous covering material.]
|