| CPC A61K 47/26 (2013.01) [A61K 9/4858 (2013.01); A61K 9/4866 (2013.01); A61K 31/5377 (2013.01); Y02A 50/30 (2018.01)] | 37 Claims |
|
8. An oral dosage form which weighs about 75 mg and comprises: 1) Compound A of the following structure:
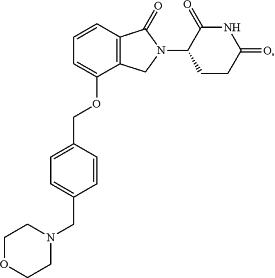 or a pharmaceutically acceptable prodrug, salt, solvate, hydrate, clathrate, stereoisomer, tautomer, or racemic mixtures thereof, at an amount that provides 0.3 mg potency of Compound A; 2) a pharmaceutically acceptable carrier or excipient [ at an amount of about 90 to about 99.9 weight percent of total weight of the oral dosage form] , wherein the carrier or excipient is a mixture of starch and lactose [ , and wherein the weight ratio of lactose to starch in the oral dosage form is from 2.1:1 to 3.9:1] ; and 3) a lubricant [ , at an amount of 0.01 to 1 weight percent of total weight of the oral dosage form, ] wherein the lubricant is stearic acid.
|