| CPC G06F 21/6254 (2013.01) [G06F 21/602 (2013.01); G16H 10/20 (2018.01)] | 17 Claims |
|
1. A system for linking trial data for a subject with other tokenized data for the subject, the system comprising:
a central management platform comprising a processor and storage;
a first client site comprising a processor and storage;
a second client site comprising a processor and storage;
a trial originator/sponsor comprising a processor and storage;
the central management platform, first client site, second client site, and trial originator/sponsor in electronic communication over a network;
the central management platform configured to:
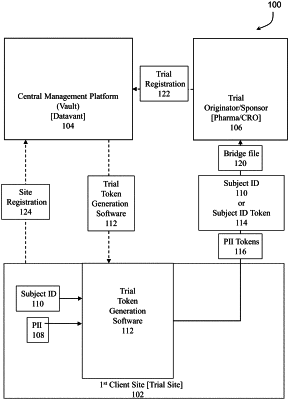
register a trial from the trial originator/sponsor;
register the first client site and the second client site participating in the trial;
generate and maintain unique encryption keys and tokenizing and encryption rules for entities participating in the trial;
provide trial token generation software to the first client site participating in the trial;
provide de-identifying software to the second client site; and
provide linking software to the trial originator/sponsor;
the first client site configured to:
receive and execute trial token generation software from the central management platform;
receive, using the trial token generation software, an identification of subject in the trial (subject ID);
receive, using the trial token generation software, personal identification information (PII) for the subject;
generate, using the trial token generation software, multiple tokens from the personal identification information (PII) for the subject, wherein the multiple personal identification information tokens are unique to the first client site and the subject;
generate, using the trial token generation software, a bridge file mapping a link between the identification of the subject in the trial (subject ID) and the multiple personal identification information tokens, wherein the bridge file links trial data for the subject tokenized with the subject identification token to other data for the subject tokenized with the multiple personal identification information tokens;
send, using the trial token generation software, the bridge file to a storage location,
collect trial data for the subject identified with the subject ID;
send the collected trial data identified with the subject ID to the trial originator/sponsor;
the second client site configured to:
collect other data for the subject including PII;
receive and execute de-identifying software from the central management platform;
tokenize, using the de-identifying software, the other data; and
send the tokenized other data to the trial originator/sponsor;
the trial originator/sponsor configured to:
register the trial with the central management platform;
receive trail data with a subject identifier from the first client site;
receive linking software from the central management platform;
receive tokenized other data from the second client site;
access the bridge file; and
link, using the linking software and the bridge file, the trial data with the tokenized other data;
wherein, only the first client site has access to the subject identification (subject ID), personal identification information (PII), and the link between the subject identification and personal identification information for the subject.
|