| CPC C08G 77/455 (2013.01) [C08G 77/08 (2013.01); C08G 77/12 (2013.01); C08G 77/20 (2013.01); C08G 77/70 (2013.01); C08G 77/80 (2013.01); C08K 3/22 (2013.01); C08K 5/3417 (2013.01); C08L 83/08 (2013.01); C08K 2003/2272 (2013.01); C08L 2201/08 (2013.01); C08L 2203/20 (2013.01); C08L 2205/03 (2013.01); C08L 2312/08 (2013.01)] | 35 Claims |
|
1. An addition curable silicone-imide composition comprising:
(A) an alkenyl functional siloxane-imide copolymer selected from a compound of the formula (I):
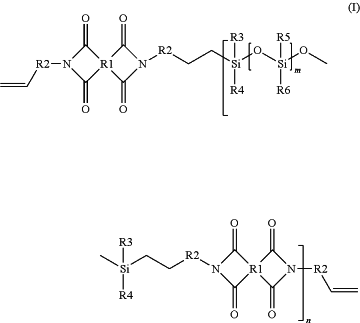 R1 is independently selected from a C5-C20 aryl, a polycyclic aryl group comprising two or more C5-C20 aryl groups, where R1 can be unsubstituted or substituted with a C1-C6 alkyl, a halogen, a haloalkyl, a hydroxy, and/or a C1-C5 alkoxy groups;
R2 is independently selected from a C1-C20 divalent hydrocarbon, a C4-C20 branched divalent hydrocarbon, or a C4-C30 cyclic containing hydrocarbon group;
R3, R4, R5, and R6 are each independently selected from a C1-C10 alkyl and a C6-C20 aryl;
m is an integer from 1 to about 200; and
n is an integer from 1 to about 30; the alkenyl functional siloxane-imide copolymer being present in the composition in an amount of from about 20 to about 50 parts by mass based on the total weight of the curable silicone composition;
(B) from about 20 parts to about 50 parts by mass of an alkenyl functional organosiloxane based on the total weight of the curable silicone composition;
(C) a polyorganohydrogensiloxane having at least two hydrogen atoms bonded to silicon atoms;
(D) a catalyst selected from precious metal catalysts selected from ruthenium, rhodium, palladium, osmium, iridium, and platinum, and complexes comprising one or more of such metals;
(E) an additive; and
(F) 0 parts by mass to about 3000 parts of a filler based on the total weight of the curable silicone composition.
|