| CPC C07K 5/1021 (2013.01) [C07K 1/02 (2013.01); C07K 1/306 (2013.01); C07K 5/10 (2013.01); C07K 5/1027 (2013.01); C07B 2200/13 (2013.01)] | 20 Claims |
|
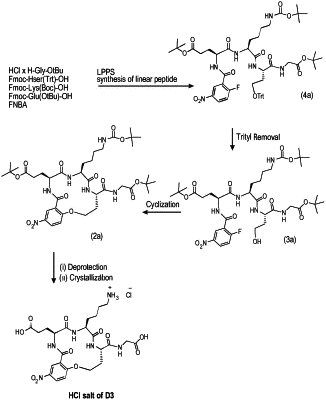
1. A method of preparing a salt of a β-turn peptidomimetic cyclic compound of formula (I)
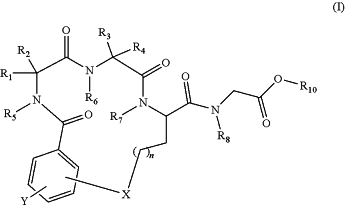 wherein:
R1 is hydrogen, C1 to C6 alkyl, aryl, or an amino acid side chain substituent of a natural or unnatural amino acid;
R3 is hydrogen, C1 to C6 alkyl, aryl, or an amino acid side chain substituent of a natural or unnatural amino acid;
R2 and R4 are independently hydrogen or C1 to C6 alkyl, or R1 and R2 together with the carbon atom to which they are attached form a cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl group, or R3 and R4 together with the carbon atom to which they are attached form a cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl group;
Y is selected from the group consisting of hydrogen, —NO2, — COOR14, —OC(R14)3, —SO3R14, and —SO2R14;
R5, R6, R7, R8, and R9 are independently hydrogen or C1 to C6 alkyl;
R10 is hydrogen, methyl, t-butyl, or a protecting group; and
each R14 is hydrogen, alkyl or aryl;
X is selected from the group consisting of O, NR9, S, P, Se, C1 to C6 alkylene, SO, SO2 and NH; and
n is 0, 1, 2, 3, 4 or 5;
the method comprising steps of:
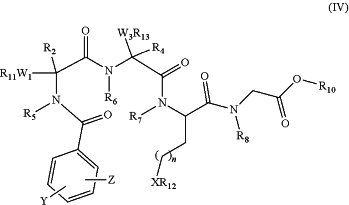
(a) providing a protected linear peptidomimetic compound of formula (IV)
 wherein:
R2, R4, R5, R6, R7, R8, and R10, X, Y, and n have the meanings defined above;
R11 and R13 are independently hydrogen or a protecting group;
R12 is a protecting group;
W1 and W3 are independently an amino acid side chain substituent of a natural or unnatural amino acid, less a hydrogen atom at the point of attachment to R11 and R13 respectively; and
Z is selected from the group consisting of F, Cl, Br and I;
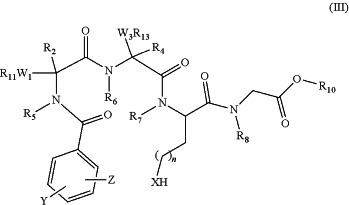
(b) selectively deprotecting the compound of formula (IV) to form a partially protected linear peptidomimetic compound of formula (III)
 wherein:
R2, R4, R5, R6, R7, R8, R10, R11, R13, W1, W3, X, Y, Z, and n have the meanings defined above;
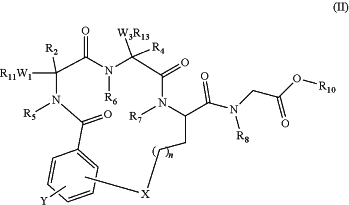
(c) cyclizing the partially protected linear peptidomimetic compound of formula (III) to form a compound of formula (II) by an intramolecular aromatic nucleophilic substitution reaction
 wherein:
R2, R4, R5, R6, R7, R8, R10, R11, R13, W1, W3, X, Y, and n have the meanings defined above; and
(d) deprotecting all amino acid side chain protecting groups in the compound of formula (II) to obtain the salt of the β-turn peptidomimetic cyclic compound of formula (I).
|