| CPC A61N 1/3752 (2013.01) [A61M 39/00 (2013.01); A61F 2002/3007 (2013.01); A61M 2039/0054 (2013.01)] | 20 Claims |
|
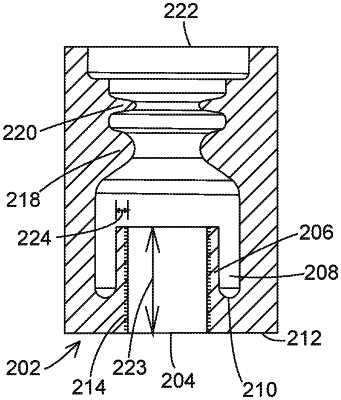
1. An implantable medical system, comprising:
a therapy delivery element having a proximal contact, a body, and a length;
a housing defining a first bore, the therapy delivery element being present within the first bore;
circuitry within the housing to at least one of deliver therapy to a patient or sense a biological signal of the patient;
an electrical connector positioned within the first bore and electrically coupled to the circuitry, with the proximal contact being in contact with the electrical connector; and
a monolithic elastic seal body coupled to the housing and having a wall that provides an outer surface of a portion of the housing, the monolithic elastic seal body comprising a first cylinder defining a seal bore, the first cylinder being positioned within the first bore and contacting the length of the therapy delivery element over a contact length of at least 0.010 inches and with an average contact pressure of no greater than (10 pounds per inch)/(contact length).
|