| CPC A61M 5/1456 (2013.01) [A61J 1/2024 (2015.05); A61M 5/24 (2013.01); A61J 1/1406 (2013.01); A61M 5/285 (2013.01)] | 21 Claims |
|
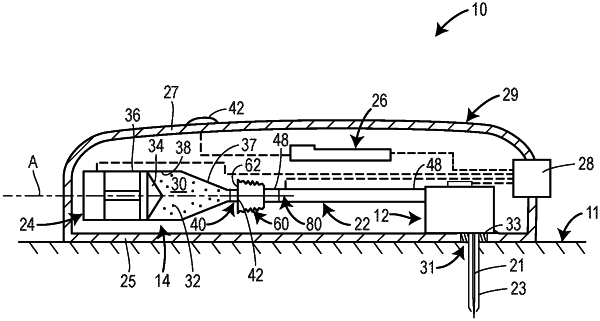
1. A drug delivery device comprising:
a housing defining an interior space;
a container disposed in the interior space and including a reservoir containing a drug and a stopper, the stopper being movable through the reservoir from a proximal end of the reservoir toward a distal end of the reservoir to expel the drug from the reservoir during operation of the drug delivery device;
a first seal member connected to the container at the distal end of the reservoir;
a first removable membrane disposed at least partially within the housing and covering an exterior surface of the first seal member to maintain sterility of the exterior surface of the first seal member prior to operation of the drug delivery device;
a fluid pathway assembly configured to establish fluid communication with the reservoir during operation of the drug delivery device and having a first end, a second end, and a fluid passage extending between the first end and the second end;
a second seal member connected to the first end of the fluid pathway assembly; and
a second removable membrane disposed at least partially within the housing and covering an exterior surface of the second seal member to maintain sterility of the exterior surface of the second seal member prior to operation of the drug delivery device.
|