| CPC A61K 39/245 (2013.01) [A61K 9/0019 (2013.01); A61K 9/5123 (2013.01); A61K 9/5146 (2013.01); A61K 39/39 (2013.01); A61K 47/6929 (2017.08); A61P 31/22 (2018.01); C07K 14/005 (2013.01); C12N 7/00 (2013.01); A61K 2039/53 (2013.01); A61K 2039/54 (2013.01); A61K 2039/545 (2013.01); A61K 2039/55516 (2013.01); A61K 2039/70 (2013.01); C07K 2319/02 (2013.01); C07K 2319/40 (2013.01); C12N 2710/16622 (2013.01); C12N 2710/16634 (2013.01)] | 9 Claims |
|
1. A messenger ribonucleic acid (mRNA) vaccine composition comprising:
(a) an mRNA polynucleotide having an open reading frame (ORF) encoding an HSV-2 glycoprotein B variant that comprises a transmembrane domain and is truncated at its C terminus, relative to a wild-type HSV-2 glycoprotein B;
(b) an mRNA polynucleotide having an ORF encoding an HSV-2 glycoprotein C variant that comprises a transmembrane domain and is truncated at its C terminus, relative to a wild-type HSV-2 glycoprotein C;
(c) an mRNA polynucleotide having an ORF encoding an HSV-2 glycoprotein D; and
(d) a lipid nanoparticle comprising 20-60% ionizable cationic lipid, 0.5-15% polyethylene glycol (PEG)-modified lipid, 25-55% cholesterol, and 5-25% neutral lipid,
wherein the ionizable lipid comprises a compound of Formula (I):
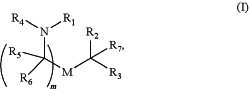 or a salt or isomer thereof, wherein:
R1 is R″M′R′ or C5-20 alkenyl;
R2 and R3 are each independently selected from C1-14 alkyl and C2-14 alkenyl;
R4 is —(CH2)nQ, wherein Q is OH and n is selected from 3, 4, and 5;
M and M′ are each independently —OC(O)— or —C(O)O—;
R5, R6, and R7 are each H;
R′ is a linear C1-12 alkyl, or C1-12 alkyl substituted with C6-9 alkyl;
R″ is C3-14 alkyl;
m is selected from 5, 6, 7, 8, 9, 10, 11, 12, and 13.
|