| CPC A61K 31/675 (2013.01) [A61K 9/0019 (2013.01); A61K 9/10 (2013.01); A61K 9/19 (2013.01); A61K 47/02 (2013.01); A61K 47/26 (2013.01); A61P 35/04 (2018.01); C07K 16/2818 (2013.01)] | 21 Claims |
|
1. A pharmaceutical combination, wherein the pharmaceutical combination is a non-fixed combination comprising:
a) a first pharmaceutical composition comprising a compound having the structure of Formula (A), or a pharmaceutically acceptable salt thereof, aluminum-containing particles, a buffering agent and one or more pharmaceutically acceptable excipients:
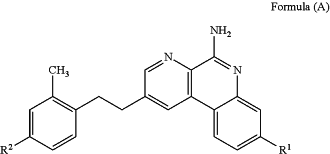 wherein:
R1 is -L1R4, -L1R5, —OL1R4, —OL1R5, CH3, —C(═O)P(O)(OH)2 or —C(═O)CF2P(O)(OH)2;
R2 is -L2R4, -L2R6, -L2L3L2R6, -L2L3R4, -L2L3L2R4, —OL2R4, —OR4, —OL2R6, —OL2L3R6, —OL2L3L2R6, —OL2L3R4, —OL2L3L2R4 or —OCH3;
each R3 is independently selected from H and fluoro;
R4 is —P(O)(OH)2,
R5 is —CF2P(O)(OH)2 or —C(O)OH;
R6 is —CF2P(O)(OH)2 or —C(O)OH;
L1 is C1-C6alkylene, C2-C6alkenylene or —((CR4R4)pO)q(CH2)p—, wherein the C1-C6alkylene and C2-C6alkenylene of L1 are substituted with 0 to 4 fluoro groups;
each L2 is independently selected from C1-C6alkylene and —((CR3R3)pO)q(CH2)p—, wherein the C1-C6alkylene of L2 is substituted with 0 to 4 fluoro groups;
L3 is arylene or a 5-6 membered heteroarylene;
each p is independently selected from 1, 2, 3, 4, 5 and 6, and
q is 1, 2, 3 or 4
and wherein
one of the one or more pharmaceutically acceptable excipients is selected from mannitol and sucrose;
the composition has a pH in the range of 6.5 to 9.0, and
the aluminum-containing particles are a suspension of aluminum hydroxide particles;
b) a second pharmaceutical composition comprising a checkpoint inhibitor selected from a CTLA-4 receptor inhibitor, a PD-1 receptor inhibitor, a LAG-3 receptor inhibitor, TIM-3 receptor inhibitor, a BTLA receptor inhibitor, a KIR receptor inhibitor, a PD-L1 inhibitor or a PD-L2 inhibitor,
and
c) a third pharmaceutical composition comprising a checkpoint inhibitor selected from a CTLA-4 receptor inhibitor, a PD-1 receptor inhibitor, a LAG-3 receptor inhibitor, TIM-3 receptor inhibitor, a BTLA receptor inhibitor, a KIR receptor inhibitor, a PD-L1 inhibitor or a PD-L2 inhibitor,
wherein the checkpoint inhibitor of the third composition is different than the checkpoint inhibitor in the second composition.
|