| CPC A61K 31/517 (2013.01) [A61K 31/5377 (2013.01); A61P 1/16 (2018.01); A61P 3/06 (2018.01); A61P 3/10 (2018.01)] | 24 Claims |
|
1. A method for lowering hepatic glucose production in a mammal, comprising administering to the mammal a therapeutically effective amount of at least one compound having a structure represented by a formula selected from:
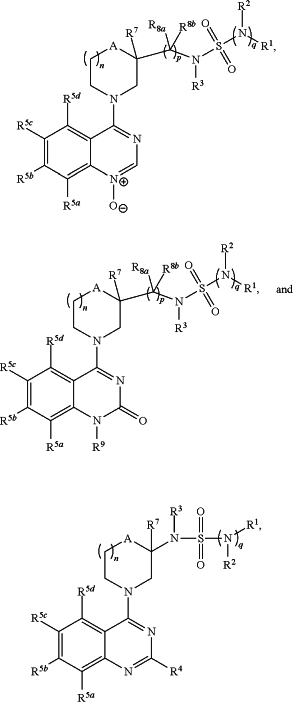 wherein n is 0, 1, or 2;
wherein p is 0, 1, 2, 3, or 4;
wherein q is 0 or 1;
wherein R1 is —NH2, —OH, C1-C4 alkyl, C1-C4 haloalkyl, C1-C4 alkoxy, C1-C4 hydroxyalkyl, C1-C4 alkylamino, (C1-C4)(C1-C4) dialkylamino, —(C1-C4 alkyl)(C1-C4 alkoxy), —(C1-C4 alkyl)CO2H, or Cy1;
wherein Cy1, when present, is C3-C6 cycloalkyl, C2-C5 heterocycloalkyl, or aryl, and is substituted with 0, 1, 2, or 3 groups independently selected from halogen, —NH2, —OH, C1-C4 alkyl, C1-C4 haloalkyl, C1-C4 alkoxy, C1-C4 alkylamino, and (C1-C4)(C1-C4) dialkylamino;
wherein R2 is hydrogen or C1-C4 alkyl, or wherein each of R1 and R2 are covalently bonded together and, together with the intermediate atoms, comprise a 3- to 6-membered heterocycloalkyl substituted with 0, 1, 2, or 3 groups independently selected from halogen, —NH2, —OH, C1-C4 alkyl, C1-C4 haloalkyl, C1-C4 alkoxy, C1-C4 alkylamino, and (C1-C4)(C1-C4) dialkylamino;
wherein R3 is hydrogen or C1-C4 alkyl, or wherein each of R1 and R3 are covalently bonded together and, together with the intermediate atoms, comprise a 5- to 7-membered heterocycloalkyl substituted with 0, 1, 2, or 3 groups independently selected from halogen, —NH2, —OH, C1-C4 alkyl, C1-C4 haloalkyl, C1-C4 alkoxy, C1-C4 alkylamino, and (C1-C4)(C1-C4) dialkylamino;
wherein R4 is hydrogen, halogen, —NH2, —OH, C1-C4 alkyl, C1-C4 alkoxy, C1-C4 alkylamino, (C1-C4)(C1-C4) dialkylamino, or Cy2;
wherein Cy2, when present, is C3-C6 cycloalkyl, C2-C5 heterocycloalkyl, or aryl, and is substituted with 0, 1, 2, or 3 groups independently selected from halogen, —NH2, —OH, C1-C4 alkyl, C1-C4 haloalkyl, C1-C4 alkoxy, C1-C4 alkylamino, and (C1-C4)(C1-C4) dialkylamino;
wherein each of R5a, R5b, R5c, and R5d is independently hydrogen, halogen, —NH2, —CN, —OH, C1-C4 alkyl, C1-C4 haloalkyl, C1-C4 alkoxy, C1-C4 haloalkoxy, C1-C4 alkylamino, (C1-C4)(C1-C4) dialkylamino, —CO2(C1-C4 alkyl), —CO2H, —CO2NH2, —NHC(O)Cy3, —NHC(O)(C1-C4 alkyl), or Cy3;
wherein Cy3, when present, is C3-C6 cycloalkyl, C2-C5 heterocycloalkyl, or aryl, and is substituted with 0, 1, 2, or 3 groups independently selected from halogen, —NH2, —OH, C1-C4 alkyl, C1-C4 haloalkyl, C1-C4 alkoxy, C1-C4 alkylamino, and (C1-C4)(C1-C4) dialkylamino;
wherein A is O, NR6a, or CHRb;
wherein R6a is hydrogen or C1-C4 alkyl; and
wherein R6b is hydrogen, C1-C4 alkyl, C1-C4 haloalkyl, C1-C4 alkoxy, C1-C4 alkylamino, (C1-C4)(C1-C4) dialkylamino, or —CO2H;
wherein R7 is hydrogen, halogen, —OH, C1-C4 alkyl, C1-C4 haloalkyl, or C1-C4 alkoxy;
wherein each occurrence of R8a and R8b, when present, is independently hydrogen, C1-C4 alkyl, C1-C4 haloalkyl, phenyl, or —CO2H;
or wherein p is 1 and each of R8a and R8b together comprise ═O; and
wherein R9 is hydrogen, C1-C4 alkyl, or Cy4,
wherein Cy4, when present, is C3-C6 cycloalkyl, C2-C5 heterocycloalkyl, or aryl, and is substituted with 0, 1, 2, or 3 groups independently selected from halogen, —NH2, —OH, C1-C4 alkyl, C1-C4 haloalkyl, C1-C4 alkoxy, C1-C4 alkylamino, and (C1-C4)(C1-C4) dialkylamino, or a pharmaceutically acceptable salt thereof.
|