| CPC G01R 33/56509 (2013.01) [G01R 33/4826 (2013.01); G01R 33/4835 (2013.01)] | 14 Claims |
|
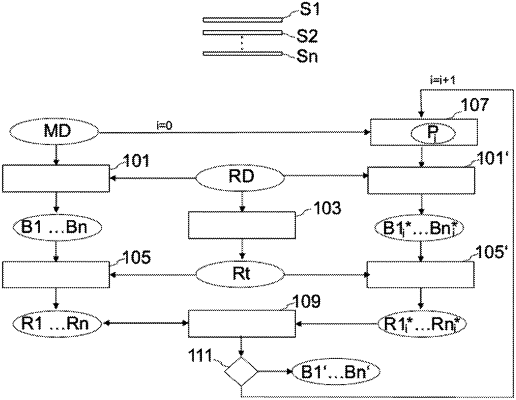
1. A method using a magnetic resonance apparatus for reconstructing single-slice image data sets from k-space measured data sets which have been acquired simultaneously from at least two slices identified with an examination object, comprising:
a) loading a k-space measured data set, which comprises measured data that has been acquired simultaneously for at least two slices from an examination object along set k-space trajectories;
b) reconstructing one reference slice image data set in each case for each of the at least two slices from the k-space measured data set;
c) determining a test region for the reference slice image data sets in which no artifacts are expected;
d) determining reference values for each of the at least two slices by analyzing the test region in the respective reference slice image data sets;
e) modifying at least one of parameters that characterize a set k-space trajectory;
f) reconstructing one test slice image data set in each case for each of the at least two slices from the k-space measured data set using the modified parameters;
g) determining test reference values that correspond with the reference values for each of the at least two slices by analyzing the test region in the respective test slice image data sets;
h) repeating steps e) to g) with a modification that differs from the modifications already tested until an abort criterion is fulfilled;
i) comparing the reference values and the test reference values in accordance with a quality criterion; and
j) storing the parameters associated with a best among the reference values and the test reference values according to the comparison,
wherein the steps a) to j) are performed by control circuitry of the magnetic resonance apparatus, and a result of the steps a) to j) are stored in a memory or displayed on an output apparatus as image data.
|