| CPC C07H 19/048 (2013.01) [C07D 307/20 (2013.01); C07H 19/20 (2013.01); C07B 2200/13 (2013.01); C07H 19/04 (2013.01)] | 17 Claims |
|
1. A method of making a compound or derivative having formula (I), or a salt, solvate, or prodrug thereof:
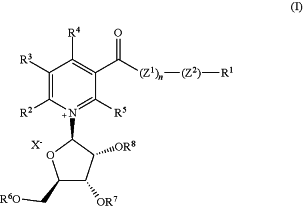 wherein X− as counterion is absent, or when X− is present, X− is selected from the group consisting of fluoride, chloride, bromide, iodide, formate, acetate, propionate, butyrate, glutamate, aspartate, ascorbate, benzoate, carbonate, citrate, carbamate, gluconate, lactate, succinate, sulfonate, malate, maleate, tartrate, glycolate, glucuronate, fumarate, pyruvate, mandelate, nitrate, panthotenate, salicylate, galactarate, galacturonate, anthralinate, 4-hydroxybenzoate, phenylacetate, pamoate, methanesulfonate, ethanesulfonate, tribromoethanesulfonate, benzenesulfonate, 2-hydroxyethanesulfonate, p-toluenesulfonate, sulfanilate, cyclohexylaminosulfonate, stearate, alginate, galactarate, galacturonate, trifluoromethanesulfonate, trichloromethanesulfonate, tribromomethanesulfonate, and trifluoroacetate;
Z1 and Z2 are independently NH or oxygen;
n is 0 or 1;
R1 is selected from the group consisting of hydrogen, substituted or unsubstituted (C1-C8)alkyl, substituted or unsubstituted (C3-C8)cycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, and substituted or unsubstituted heterocycle, vitamin B1 ester, vitamin B2 ester, vitamin B6 ester, choline ester, biotin ester, vitamin A ester, pterostilbene ester, resveratrol ester, aryl(C1-C4)alkyl, TMS, heterocycle(C1-C4)alkyl, —N(RA)—CO2RC, —N(RA)—CO2RB, —C**H—(RA)—NH2, and —C**H—(RA)—CO2RB; wherein the substituted (C1-C8)alkyl, substituted (C3-C8)cycloalkyl, substituted aryl, substituted heteroaryl, and substituted heterocycle are substituted with one to five substituents independently selected from the group consisting of -(C1-C6)alkyl, -(C2-C6)alkenyl, -(C2-C6)alkynyl, halogen, —CN, —NO2, —C(O)RC, —C(O)ORC, —C(O)NRC2, —C(═NRC)NRC2, —ORC, —OC(O)(C1-C6)alkyl, —OC(O)O(C1-C6)alkyl, —OC(O)NRC2, -(C1-C6)alkylene-NRC2, —NRC2, —NRCC(O)RC, —NRCC(O)O(C1-C6)alkyl, —NRCC(O)NRC2, —NRCSO2NRC2, —SRC, —S(O)RC, —SO2RC, —OSO2(C1-C6)alkyl, —SO2NRC2, -(C1-C6)perfluoroalkyl, and -(C1-C6)alkylene ORC;
wherein when R1 is hydrogen, Z2 is oxygen, and n is 0, the compound or derivative having formula (I) may optionally take the form of the carboxylate anion conjugate base species of the compound or derivative having formula (I), further optionally associated with a positively charged counterion selected from the group consisting of calcium, magnesium, potassium, sodium, zinc, and ammonium cations;
RA is selected from the group consisting of —H, -(C1-C6)alkyl, (CH2)3—NH—C(NH2)(═NH), —CH2C(═O)NH2, —CH2COOH, —CH2SH, —(CH2)2C(═O)—NH2, (CH2)2COOH, —CH2-(2-imidazolyl), —CH(CH3)—CH2—CH3, —CH2CH(CH3)2, —(CH2)4—NH2, —(CH2)2—S—CH3, phenyl, —CH2-phenyl, —CH2—OH, —CH(OH)—CH3, —CH2-(3-indolyl), —CH2-(4-hydroxyphenyl), —CH(CH3)2, —NH2, and —CH2—CH3;
each RB is independently hydrogen or -(C1-C8)alkyl;
each RC is independently selected from the group consisting of hydrogen, -(C1-C8)alkyl, substituted or unsubstituted pyridyl, and substituted or unsubstituted 1,4-dihydropyridyl; wherein the substituted pyridyl and substituted 1,4-dihydropyridyl are substituted with one to five substituents independently selected from the group consisting of (C1-C6)alkyl, -(C2-C6)alkenyl, -(C2-C6)alkynyl, halogen, —CN, —NO2, —C(O)RB, —C(O)ORB, —C(O)NRB2, —C(═NRB)NRB2, —ORB, —OC(O)(C1-C6)alkyl, —OC(O)O(C1-C6)alkyl, —OC(O)NRB2, -(C1-C6)alkylene-NRB2, —NRB2, —NRBC(O)RB, —NRBC(O)O(C1-C6)alkyl, —NRBC(O)NRB2, —NRBSO2NRB2, —SRB, —S(O)RB, —SO2RB, —OSO2(C1-C6)alkyl, —SO2NRB2, -(C1-C6)perfluoroalkyl, and -(C1-C6)alkylene-ORB;
R2, R3, R4, and R5 are each independently selected from the group consisting of hydrogen, -(C1-C6)alkyl, -(C2-C6)alkenyl, -(C2-C6)alkynyl, halogen, —CN, —NO2, —C(O)RC, —C(O)ORC, —C(O)NRC2, —C(═NRC)NRC2, —ORC, —OC(O)(C1-C6)alkyl, —OC(O)O(C1-C6)alkyl, —OC(O)NRC2, -(C1-C6)alkylene-NRC2, —NRC2, —NRCC(O)RC, —NRCC(O)O(C1-C6)alkyl, —NRCC(O)NRC2, —NRCSO2NRC2, —SRC, —S(O)RC, —SO2RC, —OSO2(C1-C6)alkyl, —SO2NRC2, -(C1-C6)perfluoroalkyl, and -(C1-C6)alkylene-ORC;
R6 is selected from the group consisting of hydrogen, —C(O)R′, —C(O)OR′, —C(O)NHR′, substituted or unsubstituted (C1-C5)alkyl, substituted or unsubstituted (C3-C8)cycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, and substituted or unsubstituted heterocycle, vitamin B1 ester, vitamin B2 ester, vitamin B6 ester, choline ester, biotin ester, vitamin A ester, resveratrol ester, glutathione ester, glutathione disulfide ester, aryl(C1-C4)alkyl, heterocycle(C1-C4)alkyl, —N(RA)—CO2RC, —N(RA)—CO2RB, —C**H—(RA)—NH2, and —C**H—(RA)—CO2RB; wherein the substituted (C1-C8)alkyl, substituted (C3-C8)cycloalkyl, substituted aryl, substituted heteroaryl, and substituted heterocycle are substituted with one to five substituents independently selected from the group consisting of -(C1-C6)alkyl, -(C2-C6)alkenyl, -(C2-C6)alkynyl, halogen, —CN, —NO2, —C(O)RC, —C(O)ORC, —C(O)NRC2, —C(═NRC)NRC2, —ORC, —OC(O)(C1-C6)alkyl, —OC(O)O(C1-C6)alkyl, —OC(O)NRC2, -(C1-C6)alkylene-NRC2, —NRC2, —NRCC(O)RC, —NRC(O)O(C1-C6)alkyl, —NRCC(O)NRC2, —NRCSO2NRC2, —SRC, —S(O)RC, —SO2RC, —OSO2(C1-C6)alkyl, —SO2NRC2, -(C1-C6)perfluoroalkyl, and -(C1-C6)alkylene ORC;
R′ is selected from the group consisting of hydrogen, substituted or unsubstituted (C1-C8)alkyl, substituted or unsubstituted (C3-C8)cycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted heterocycle, vitamin B1 ester, vitamin B2 ester, vitamin B6 ester, choline ester, biotin ester, vitamin A ester, resveratrol ester, aryl(C1-C4)alkyl, heterocycle(C1-C4)alkyl, —N(RA)—CO2RC, —N(RA)—CO2RB, —C**H—(RA)—NH2, and —C**H—(RA) CO2RB; wherein the substituted (C1-C8)alkyl, substituted (C3-C8)cycloalkyl, substituted aryl, substituted heteroaryl, and substituted heterocycle are substituted with one to five substituents independently selected from the group consisting of -(C1-C6)alkyl, -(C2-C6)alkenyl, -(C2-C6)alkynyl, halogen, —CN, —NO2, —C(O)RC, —C(O)ORC, —C(O)NRC2, —C(═NRC)NRC2, —ORC, —OC(O)(C1-C6)alkyl, —OC(O)O(C1-C6)alkyl, —OC(O)NRC2, -(C1-C6)alkylene-NRC2, —NRC2, —NRCC(O)RC, —NRC(O)O(C1-C6)alkyl, —NRCC(O)NRC2, —NRCSO2NRC2, —SRC, —S(O)RC, —SO2RC, —OSO2(C1-C6)alkyl, —SO2NRC2, -(C1-C6)perfluoroalkyl, and -(C1-C6)alkylene-ORC;
R7 and R8 are independently selected from the group consisting of hydrogen, —C(O)R′, —C(O)OR′, —C(O)NHR′, substituted or unsubstituted (C1-C8)alkyl, substituted or unsubstituted (C3-C8)cycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted heterocycle, substituted or unsubstituted aryl(C1-C4)alkyl, and substituted or unsubstituted heterocycle(C1-C4)alkyl; wherein the substituted (C1-C8)alkyl, substituted (C3-C8)cycloalkyl, substituted aryl, substituted heteroaryl, substituted heterocycle, substituted aryl(C1-C4)alkyl, and substituted heterocycle(C1-C4)alkyl are substituted with one to five substituents independently selected from the group consisting of -(C1-C6)alkyl, -(C2-C6)alkenyl, -(C2-C6)alkynyl, halogen, —CN, —NO2, —C(O)RC, —C(O)ORC, —C(O)NRC2, —C(═NRC)NRC2, —ORC, —OC(O)(C1-C6)alkyl, —OC(O)O(C1-C6)alkyl, —OC(O)NRC2, -(C1-C6)alkylene-NRC2, —NRC2, —NRCC(O)RC, —NRC(O)O(C1-C6)alkyl, —NRC(O)NRC2, —NRCSO2NRC2, —SRC, —S(O)RC, —SO2RC, —OSO2(C1-C6)alkyl, —SO2NRC2, -(C1-C6)perfluoroalkyl, and -(C1-C6)alkylene-ORC;
provided that the absolute configuration of C** is R or S, or a mixture of R and S;
and wherein the anomeric carbon configuration is alpha- or beta-, or a mixture of alpha-and beta-anomers;
comprising the steps of:
(a) providing a compound or derivative having formula (2), or salt thereof:
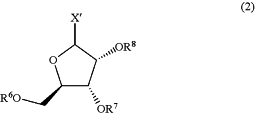 wherein X′ is selected from the group consisting of fluoro, chloro, bromo, iodo, HCO2, acetoxy, propionoxy, butyroxy, glutamyloxy, aspartyloxy, ascorbyloxy, benzoyloxy, 4-hydroxybenzoxy, phenylacetoxy, pamoatoxy, methanesulfoxy, ethanesulfoxy, benzenesulfoxy, HOCO2, citryloxy, carbamyloxy, gluconyloxy, lactyloxy, succinyloxy, sulfoxy, malateoxy, maleateoxy, tartaroxy, glycoloxy, glucoronoxy, fumaroxy, pyruvoxy, mandeloxy, panthothenoxy, salicyloxy, galactaroxy, galacturonoxy, 2-hydroxyethanesulfoxy, p-toluenesulfoxy, sulfaniloxy, cyclohexylaminosulfoxy, steroxy, alginoxy, beta-hydroxybutyroxy, trifluoromethanesulfoxy, trichloromethanesulfoxy, tribromomethanesulfoxy, and trifluoroacetoxy;
R6 is selected from the group consisting of hydrogen, —C(O)R′, —C(O)OR′, —C(O)NHR′, substituted or unsubstituted (C1-C8)alkyl, substituted or unsubstituted (C3-C8)cycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, and substituted or unsubstituted heterocycle, vitamin B1 ester, vitamin B2 ester, vitamin B6 ester, choline ester, biotin ester, vitamin A ester, resveratrol ester, glutathione ester, glutathione disulfide ester, aryl(C1-C4)alkyl, heterocycle(C1-C4)alkyl, —N(RA)—CO2RC, —N(RA)—CO2RB, —C**H—(RA)—NH2, and C**H—(RA) CO2RB; wherein the substituted (C1-C5)alkyl, substituted (C3-C8)cycloalkyl, substituted aryl, substituted heteroaryl, and substituted heterocycle are substituted with one to five substituents independently selected from the group consisting of -(C1-C6)alkyl, -(C2-C6)alkenyl, -(C2-C6)alkynyl, halogen, —CN, —NO2, —C(O)RC, —C(O)ORoC, —C(O)NRC2, —C(═NRC)NRC2, —ORC, —OC(O)(C1-C6)alkyl, —OC(O)O(C1-C6)alkyl, —OC(O)NRC2, -(C1-C6)alkylene-NRC2, —NRC2, —NRCC(O)RC, —NRCC(O)O(C1-C6)alkyl, —NRCC(O)NRC2, —NRCSO2NRC2, —SRC, —S(O)RC, —SO2RC, —OSO2(C1-C6)alkyl, —SO2NRC2, -(C1-C6)perfluoroalkyl, and -(C1-C6)alkylene-ORC;
R′ is selected from the group consisting of hydrogen, substituted or unsubstituted (C1-C8)alkyl, substituted or unsubstituted (C3-C8)cycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted heterocycle, vitamin B1 ester, vitamin B2 ester, vitamin B6 ester, choline ester, biotin ester, vitamin A ester, resveratrol ester, aryl(C1-C4)alkyl, heterocycle(C1-C4)alkyl, —N(RA)—CO2RC, —N(RA)—CO2RB, —C**H—(RA)—NH2, and C**H—(RA)—CO2RB; wherein the substituted (C1-C8)alkyl, substituted (C3-C8)cycloalkyl, substituted aryl, substituted heteroaryl, and substituted heterocycle are substituted with one to five substituents independently selected from the group consisting of -(C1-C6)alkyl, (C2-C6)alkenyl, -(C2-C6)alkynyl, halogen, —CN, —NO2, —C(O)RC, —C(O)ORC, —C(O)NRC2, —C(═NRC)NRC2, —ORC, —OC(O)(C1-C6)alkyl, —OC(O)O(C1-C6)alkyl, —OC(O)NRC2, -(C1-C6)alkylene-NRC2, —NRC2, —NRCC(O)RC, —NRC(O)O(C1-C6)alkyl, —NRCC(O)NRC2, —NRCSO2NRC2, —SRC, —S(O)RC, —SO2RC, —OSO2(C1-C6)alkyl, —SO2NRC2, -(C1-C6)perfluoroalkyl, and -(C1-C6)alkylene-ORC;
RA is selected from the group consisting of —H, -(C1-C6)alkyl, —(CH2)3—NH—C(NH2)(═NH), —CH2C(═O)NH2, —CH2COOH, —CH2SH, —(CH2)2C(═O)—NH2, —(CH2)2COOH, —CH2-(2-imidazolyl), —CH(CH3)—CH2—CH3, —CH2CH(CH3)2, —(CH2)4—NH2, —(CH2)2—S—CH3, phenyl, —CH2-phenyl, —CH2—OH, —CH(OH)—CH3, —CH2-(3-indolyl), —CH2-(4-hydroxyphenyl), —CH(CH3)2, —NH2, and —CH2—CH3;
each RB is independently hydrogen or -(C1-C8)alkyl;
each RC is independently selected from the group consisting of hydrogen, -(C1-C8)alkyl, substituted or unsubstituted pyridyl, and substituted or unsubstituted 1,4-dihydropyridyl; wherein the substituted pyridyl and substituted 1,4-dihydropyridyl are substituted with one to five substituents independently selected from the group consisting of -(C1-C6)alyl, -(C2-C6)alkenyl, -(C2-C6)alkynyl, halogen, —CN, —NO2, —C(O)RB, —C(O)ORB, —C(O)NRB2, —C(═NRB)NRB2, —ORB, —OC(O)(C1-C6)alkyl, —OC(O)O(C1-C6)alkyl, —OC(O)NRB2, -(C1-C6)alkylene-NRB2, —NRB2, —NRBC(O)RB, —NRBC(O)O(C1-C6)alkyl, —NRBC(O)NRB2, —NRBSO2NRB2, —SRB, —S(O)RB, —SO2RB, —OSO2(C1-C6)alkyl, —SO2NRB2, -(C1-C6)perfluoroalkyl, and (C1-C6)alkylene-ORB;
R7 and R8 are independently selected from the group consisting of hydrogen, —C(O)R′, —C(O)OR′, —C(O)NHR′, substituted or unsubstituted (C1-C8)alkyl, substituted or unsubstituted (C3-C8)cycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted heterocycle, substituted or unsubstituted aryl(C1-C4)alkyl, and substituted or unsubstituted heterocycle(C1-C4)alkyl; wherein the substituted (C1-C8)alkyl, substituted (C3-C8)cycloalkyl, substituted aryl, substituted heteroaryl, substituted heterocycle, substituted aryl(C1-C4)alkyl, and substituted heterocycle(C1-C4)alkyl are substituted with one to five substituents independently selected from the group consisting of -(C1-C6)alkyl, -(C2-C6)alkenyl, -(C2-C6)alkynyl, halogen, —CN, —NO2, —C(O)RC, —C(O)ORc, —C(O)NRC2, —C(═NRc)NRC2, —ORC, —OC(O)(C1-C6)alkyl, —OC(O)O(C1-C6)alkyl, —OC(O)NRC2, -(C1-C6)alkylene-NRC2, —NRC2, —NRCC(O)RC, —NRCC(O)O(C1-C6)alkyl, —NRCC(O)NRC2, —NRCSO2NRC2, —SRC, —S(O)RC, —SO2RC, —OSO2(C1-C6)alkyl, —SO2NRC2, -(C1-C6)perfluoroalkyl, and -(C1-C6)alkylene-ORC;
provided that the absolute configuration of C** is R or S, or a mixture of R and S;
(b) treating the compound or derivative having formula (2), or salt thereof, with a stoichiometrically equivalent amount of a compound or derivative having formula (1), or a salt thereof, optionally wherein each R1 is a trimethylsilyl (“TMS”) group;
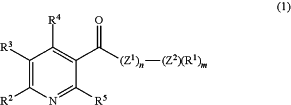 wherein Z1 and Z2 are independently nitrogen or oxygen;
m is 1 or 2;
n is 0 or 1;
each R1 is independently selected from the group consisting of hydrogen, substituted or unsubstituted (C1-C8)alkyl, substituted or unsubstituted (C3-C8)cycloalkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, and substituted or unsubstituted heterocycle, pterostilbene ester, vitamin B1 ester, vitamin B2 ester, vitamin B6 ester, choline ester, biotin ester, vitamin A ester, resveratrol ester, aryl(C1-C4)alkyl, heterocycle(C1-C4)alkyl, TMS, —N(RA)—CO2RC, —N(RA)—CO2RB, —C**H—(RA)—NH2, and —C**H—(RA)—CO2RB; wherein the substituted (C1-C8)alkyl, substituted (C3-C8)cycloalkyl, substituted aryl, substituted heteroaryl, and substituted heterocycle are substituted with one to five substituents independently selected from the group consisting of (C1-C6)alkyl, -(C2-C6)alkenyl, -(C2-C6)alkynyl, halogen, —CN, —NO2, —C(O)RC, —C(O)ORC, —C(O)NRC2, —C(═NRC)NRC2, —ORC, —OC(O)(C1-C6)alkyl, —OC(O)O(C1-C6)alkyl, —OC(O)NRC2, -(C1-C6)alkylene-NRC2, —NRC2, —NRCC(O)RC, —NRCC(O)O(C1-C6)alkyl, —NRCC(O)NRC2, —NRCSO2NRC2, —SRC, —S(O)RC, —SO2RC, —oso2(C1-C6)alkyl, —SO2NRC2, -(C1-C6)perfluoroalkyl, and -(C1-C6)alkylene-ORC;
wherein when each R1 is hydrogen, Z2 is oxygen, m is 1, and n is 0, the compound or derivative having formula (1) may optionally take the form of the carboxylate anion conjugate base species of the compound or derivative having formula (1), further optionally associated with a positively charged counterion selected from the group consisting of calcium, magnesium, potassium, sodium, zinc, and ammonium cations;
RA is selected from the group consisting of —H, -(C1-C6)alkyl, —(CH2)3—NH—C(NH2)(═NH), —CH2C(═O)NH2, —CH2COOH, —CH2SH, —(CH2)2C(═O)—NH2, —(CH2)2COOH, —CH2-(2-imidazolyl), —CH(CH3)—CH2—CH3, —CH2CH(CH3)2, —(CH2)4—NH2, —(CH2)2—S—CH3, phenyl, —CH2-phenyl, —CH2—OH, —CH(OH)—CH3, —CH2-(3-indolyl), —CH2-(4-hydroxyphenyl), —CH(CH3)2, —NH2, and —CH2—CH3;
each RB is independently hydrogen or -(C1-C8)alkyl;
each RC is independently selected from the group consisting of hydrogen, -(C1-C8)alkyl, substituted or unsubstituted pyridyl, and substituted or unsubstituted 1,4-dihydropyridyl; wherein the substituted pyridyl and substituted 1,4-dihydropyridyl are substituted with one to five substituents independently selected from the group consisting of -(C1-C6)alkyl, -(C2-C6)alkenyl, -(C2-C6)alkynyl, halogen, —CN, —NO2, —C(O)RB, —C(O)ORB, —C(O)NRB2, —C(═NRB)NRB2, —ORB, —OC(O)(C1-C6)alkyl, —OC(O)O(C1-C6)alkyl, —OC(O)NRB2, -(C1-C6)alkylene-NRB2, —NRB2, —NRBC(O)RB, —NRBC(O)O(C1-C6)alkyl, —NRBC(O)NRB2, —NRBSO2NRB2, —SRB, —S(O)RB, —SO2RB, —OSO2(C1-C6)alkyl, —SO2NRB2, -(C1-C6)perfluoroalkyl, and (C1-C6)alkylene-ORB;
R2, R3, R4, and R5 are each independently selected from the group consisting of hydrogen, -(C1-C6)alkyl, -(C2-C6)alkenyl, -(C2-C6)alkynyl, halogen, —CN, —NO2, —C(O)RC, —C(O)ORC, —C(O)NRC2, —C(═NRC)NRC2, —ORC, —OC(O)(C1-C6)alkyl, —OC(O)O(C1-C6)alkyl, —OC(O)NRC2, -(C1-C6)alkylene-NRC2, —NRC2, —NRCC(O)RC, —NRC(O)O(C1-C6)alkyl, —NRC(O)NRC2, —NRCSO2NRC2, —SRC, —S(O)RC, —SO2RC, —OSO2(C1-C6)alkyl, —SO2NRC2, -(C1-C6)perfluoroalkyl, and -(C1-C6)alkylene-ORC;
provided that the absolute configuration of C** is R or S, or a mixture of R and S;
(c) processing the compound or derivative having formula (2), or salt thereof, and the compound or derivative having formula (1), or salt thereof, optionally wherein each R1 is a TMS group, by liquid-assisted mixing, milling, grinding, or extruding so as to produce the compound or derivative having formula (I), or salt, solvate, or prodrug thereof; and
(d)isolating the compound or derivative having formula (I), or salt, solvate, or prodrug thereof.
|