| CPC C07D 498/06 (2013.01) [A61K 45/06 (2013.01); A61P 31/04 (2018.01)] | 17 Claims |
|
1. An antibiotic compound of formula (A1):
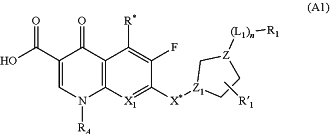 or a pharmaceutically acceptable salt, solvate, tautomer, or combination thereof, wherein:
X1 is C—RB, and RA and RB together with the atoms to which they are attached form a 6-membered ring wherein from RA to RB is a —CH(CH3)—CH2—O— linking group;
R* is H or NH2;
either Z1 is CH and X* is —NR′—; or Z1 is N and X* is absent;
L1 is —NH— or —N(CH3)—;
n is 1;
Z is N or C—H;
R′1 is H, C1-6 alkyl or —(CH2)t—C(═O)—OR′;
R1 is Ar1;
Ar1 is a six-membered ring; and the Ar1 group may be optionally substituted with 1, 2 or 3 substituents selected from the group consisting of —C1-6 alkyl, -halo, —(CH2)t—OR′, —(CH2)r C(═O)—OR′, oxo, —(CH2)t—NR′R″, —NO2, —NR′-(cyclopropyl), -(cyclopropyl), —NR′—(CH2)t—NR′R″, —C(═NR′)—NR′R″, —(CH2)t—NR′—C(═NR′)—NR′R″, —CH2—CH═CH2, —CH═CH—(C1-6 alkyl), —CH═CH—CN, —SO2—NR′R″ and —SO2NR′—(CH2)t—Ar2;
each t is independently 0, 1, 2, 3, 4 or 5;
each Ar2 is independently C5-9 heteroaryl; and
each R′ and R″ is independently H or C1-6 alkyl.
|