| CPC C07D 403/14 (2013.01) [A61P 35/00 (2018.01); C07D 401/14 (2013.01); C07D 403/12 (2013.01); C07D 405/14 (2013.01); C07D 413/14 (2013.01); C07D 471/08 (2013.01)] | 27 Claims |
|
1. A compound of Formula (I):
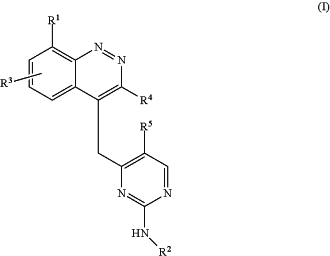 or a pharmaceutically acceptable salt thereof, wherein:
R1 is a C1-C5 alkyl, C3-C5 carbocycle, or a halogen;
R2 is an aryl of at least 6 carbon atoms or nitrogen-containing heteroaryl of at least 6 atoms, optionally substituted with one or more of:
(i) one or more halogens;
(ii) a C1-C6 alkyl optionally substituted with a hydroxyl or one or more halogen wherein, when selected to be an alkyl larger than C3, the alkyl is present at a position on the aryl or heteroaryl of R2 which is meta- or para- to the amino bond to the aryl or heteroaryl of R2;
(iii) a sulfonamide;
(iv) a monocyclic, bicyclic, or spirocyclic carbocycle which is optionally substituted with a hydroxyl, one or more halogen, or one or more linear, branched, or cyclic alkyl moieties of up to 6 carbon atoms which are optionally substituted with hydroxy or one or more halogen, wherein said carbocycle is attached to the aryl or heteroaryl of R2 by a single bond or a methylene or ethylene linker and wherein, when present and selected to be a carbocycle larger than cyclopropyl, the carbocycle is at a position on the aryl or heteroaryl of R2 which is meta- or para- to the amino bond to the aryl or heteroaryl of R2; or
(v) a monocyclic, bicyclic or spirocyclic heterocycle which may contain up to 3 heteroatoms which are selected independently from N and O, and which is optionally and independently substituted with one or more C1-C6 alkyl or C3-C6 carbocycle which are optionally substituted with hydroxy or one or more halogen, wherein said heterocycle is attached to the aryl or heteroaryl of R2 by a single bond or a methylene or ethylene linker and wherein, when present, said heterocycle is at a position on the aryl of R2 which is meta- or para- to the amino bond to said aryl;
R3 is —H, —F, or —Cl;
R4 is —H, a halogen, or a C1-C3 alkyl or cyclopropyl optionally substituted with one or more —F; and
R5 is —H, —F, or a C1-C3 alkyl or cyclopropyl optionally substituted with one or more —F.
|