| CPC C07D 401/04 (2013.01) [C07D 215/38 (2013.01); C07D 217/06 (2013.01); C07D 217/26 (2013.01); C07D 401/12 (2013.01); C07D 409/04 (2013.01); C07D 417/04 (2013.01); A61K 9/0019 (2013.01); A61K 9/0053 (2013.01)] | 15 Claims |
|
1. A compound of the formula:
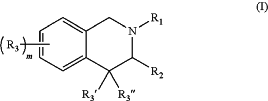 wherein:
R1 is —C(O)R4, —S(O)2R4, —S(O)R4, or —CH2R4; wherein:
R4 is aryl(C≤12), heteroaryl(C≤12), or a substituted version of either of these groups;
R2 is —(CH2)nC(O)NR5R6; wherein:
R5 and R6 are each independently hydrogen or alkyl(C≤12), aryl(C≤12), aralkyl(C≤12), heteroaryl(C≤12), heteroaralkyl(C≤12), or a substituted version of any of these five groups;
n is 0, 1, 2, or 3;
R3 is aryl(C≤12), substituted aryl(C≤12), heteroaryl(C≤12), or substituted heteroaryl(C≤12);
R3′ and R3″ are each independently hydrogen, alkyl(C≤8), or substituted alkyl(C≤8); and
m is 1, 2, 3, or 4;
or a pharmaceutically acceptable salt thereof.
|
|
14. A pharmaceutical composition comprising:
(A) a compound of the formula:
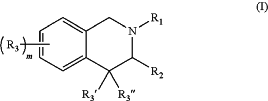 wherein:
R1 is —C(O)R4, —S(O)2R4, —S(O)R4, or —CH2R4; wherein:
R4 is aryl(C≤12), heteroaryl(C≤12), or a substituted version of either of these groups;
R2 is —(CH2)nC(O)NR5R6; wherein:
R5 and R6 are each independently hydrogen or alkyl(C≤12), aryl(C≤12), aralkyl(C≤12), heteroaryl(C≤12), heteroaralkyl(C≤12), or a substituted version of any of these five groups;
n is 0, 1, 2, or 3;
R3 is aryl(C≤12), substituted aryl(C≤12), heteroaryl(C≤12), or substituted heteroaryl(C≤12);
R3′ and R3″ are each independently hydrogen, alkyl(C≤8), or substituted alkyl(C≤8); and
m is 1, 2, 3, or 4;
or a pharmaceutically acceptable salt thereof;
and
(B) an excipient,
wherein the pharmaceutical composition is formulated for administration: orally, intraadiposally, intraarterially, intraarticularly, intracranially, intradermally, intralesionally, intramuscularly, intranasally, intraocularly, intrapericardially, intraperitoneally, intrapleurally, intraprostatically, intrarectally, intrathecally, intratracheally, intratumorally, intraumbilically, intravaginally, intravenously, intravesicularly, intravitreally, liposomally, locally, mucosally, parenterally, rectally, subconjunctivally, subcutaneously, sublingually, topically, transbuccally, transdermally, vaginally, in crèmes, in lipid compositions, via a catheter, via a lavage, via continuous infusion, via infusion, via inhalation, via injection, via local delivery, or via localized perfusion.
|