| CPC C07C 67/08 (2013.01) [C07C 2601/10 (2017.05)] | 5 Claims |
|
1. A process for preparing a (1S,2R)-(2-hydroxy-3,5,5-trimethyl-3-cyclopentenyl)methyl carboxylate compound of the following general formula (S,R)-(2):
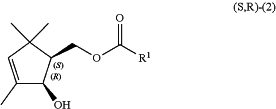 wherein R1 represents a monovalent hydrocarbon group having 1 to 6 carbon atoms, and
a bold wedged bond represents the absolute configuration, and
a (1R,2S)-(2-acetoxy-3,5,5-trimethyl-3-cyclopentenyl)methyl carboxylate compound of the following general formula (R,S)-(3):
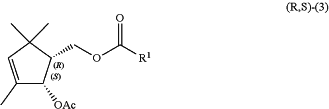 wherein R1 is as defined above, a hashed wedged bond represents the absolute configuration, and Ac represents an acetyl group,
the process comprising:
subjecting a (1RS,2SR)-(2-hydroxy-3,5,5-trimethyl-3-cyclopentenyl)methyl carboxylate compound of the following general formula (RS,SR)-(2):
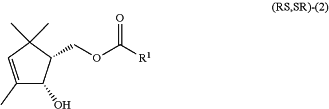 wherein R1 is as defined above, and a hashed unwedged bond represents a relative configuration,
to a kinetic resolution reaction with a lipase in the presence of vinyl acetate to obtain the (1S,2R)-(2-hydroxy-3,5,5-trimethyl-3-cyclopentenyl)methyl carboxylate compound ((S,R)-(2)) and the (1R,2S)-(2-acetoxy-3,5,5-trimethyl-3-cyclopentenyl)methyl carboxylate compound ((R,S)-(3)).
|