| CPC A61M 5/14276 (2013.01) [A61M 5/14212 (2013.01); A61M 5/1582 (2013.01); A61M 5/16809 (2013.01); A61M 5/172 (2013.01); A61M 25/0026 (2013.01); A61M 27/006 (2013.01); A61M 2005/14208 (2013.01); A61M 2205/3553 (2013.01); A61M 2205/52 (2013.01)] | 17 Claims |
|
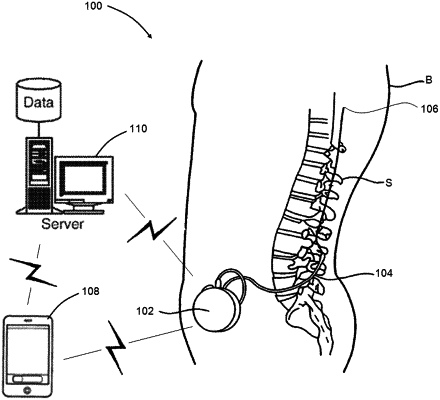
1. An implantable medical pump comprising:
a pump housing configured to be subcutaneously implanted into a body of a patient;
a medicament reservoir contained within the pump housing, wherein the medicament reservoir is configured to contain a medicament;
at least one catheter extending from the pump housing to a target location in the body of the patient, wherein the at least one catheter is in fluid communication with the medicament reservoir;
a pump mechanism; and
a processor configured to control the pump mechanism to:
deliver the medicament from the medicament reservoir to the target location;
aspirate a fluid from the target location back into the medicament reservoir; and
deliver the fluid from the medicament reservoir back to the target location.
|