| CPC A61K 38/1866 (2013.01) [A61K 47/65 (2017.08); A61K 47/6803 (2017.08); A61K 47/6835 (2017.08); A61K 47/6889 (2017.08); A61P 35/00 (2018.01); A61K 2039/572 (2013.01)] | 47 Claims |
|
1. An antibody conjugate comprising an antibody, or an antigen-binding fragment thereof, covalently linked to a drug payload (PA) and further covalently linked to an immunomodulatory payload (IM) wherein
the drug payload is a residue of a cytotoxic compound selected from the group consisting of an alkylating agent, a DNA-crosslinking agent, an anti-tumor antibiotic, an anti-metabolite, an anti-mitotic agent, a histone-deacetylase (HDAC) inhibitor, a telomerase inhibitor, and an immunogenic cell death agent; and
the immunomodulatory payload is a residue of an immunomodulatory compound selected from the group consisting of a kinase inhibitor, a growth factor inhibitor, a Calcineurin inhibitor, a CRAC inhibitor, a PARPl antagonist, a PPARγ agonist, a Kvl.3 antagonist, a PP2A agonist, a MYD88 inhibitor, a BCL-2 inhibitor, an Adenosine A2A receptor (A2ar) agonist, a Toll-like receptor 7/8 (TLR7/8) agonist, a Toll-like receptor 4 (TLR4) agonist, a Toll-like receptor 9 (TLR9) agonist, a calcium-activated potassium channel (Kca3.1) agonist, a TGF˜R1 inhibitor, a TGF-R2 inhibitor, a GLi 1 inhibitor, a tankyrase (TNKS) antagonist, a Traf2 and Nck-interacting kinase (TNIK) antagonist, an imide, and a vitamin D receptor (VDR) agonist; and wherein
(i) the antibody or antigen-binding fragment thereof comprises a non-natural amino acid at position K42 and at least one non-natural amino acid at position Y180 or F404 that are covalently linked to the immunomodulatory payload or to the drug payload; and/or
(ii) the antibody or antigen-binding fragment thereof comprises one or more glutamine (Q) residues that is covalently linked to the immunomodulatory payload or to the drug payload; and/or wherein
(iii) the drug payload or the immunomodulatory payload is linked to the antibody, or antigen-binding fragment, via one or more linkers and the one or more linkers comprise a release trigger group, wherein the release trigger group is a β-glucuronidase-cleavable β-glucuronide according to the structure
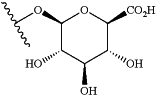 wherein
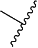 indicates the atoms in the antibody drug conjugate to which the β-glucuronidase-cleavable β-glucuronide is bonded.
|