| CPC A61K 38/13 (2013.01) [A61K 9/0053 (2013.01); A61K 9/0078 (2013.01); A61K 9/127 (2013.01); A61K 9/19 (2013.01); A61K 45/06 (2013.01); A61K 47/26 (2013.01)] | 21 Claims |
|
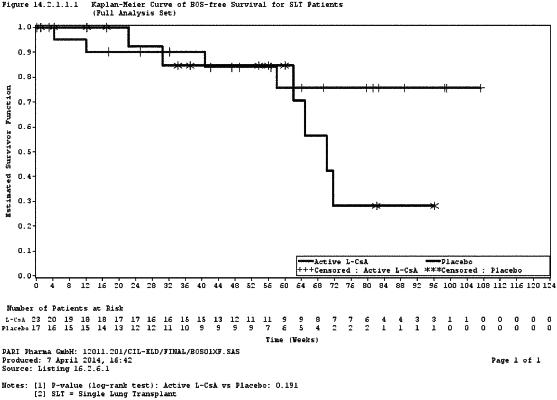
1. A method of preventing or treating pulmonary chronic graft rejection in single lung transplanted patients comprising administering to a patient in need thereof a liposomal cyclosporine liquid formulation as an aerosol for inhalation;
wherein the formulation contains the cyclosporine at a concentration of 1 mg/mL up to 5 mg/ml and wherein the volume of a unit dose of the formulation is 1 to 3 mL,
wherein the patient is a human patient of higher age, and wherein the patient is co-administered one or more active ingredients used in standard immunosuppressive therapy after lung transplantation, wherein the one or more active ingredients constitute a standard of care systemic immunosuppression regimen.
|