| CPC A61K 31/4192 (2013.01) [A61K 9/0053 (2013.01); A61K 31/4178 (2013.01); A61K 31/496 (2013.01); A61K 39/39558 (2013.01); A61K 45/06 (2013.01); A61P 35/00 (2018.01); C07K 16/2827 (2013.01); C07K 16/3015 (2013.01); A61K 2039/505 (2013.01); A61K 2039/54 (2013.01); C07K 2317/76 (2013.01)] | 10 Claims |
|
1. A method for treating a subject having a solid tumor expressing PD-1, said method comprising administering to the subject in need thereof a therapeutically effective amount of a CCR1 chemokine receptor antagonist, and a therapeutically effective amount of a PD-1 inhibitor,
wherein the CCR1 chemokine receptor antagonist has the formula (IIIb1a):
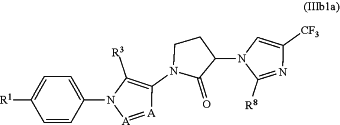 wherein each A is N or CH and at least one A is N; R1 is halogen; R3 is selected from the group consisting of C1-8 alkyl, C3-8 cycloalkyl and C2-8 alkenyl; and R8 is C1-8 alkyl; or a pharmaceutically acceptable salt thereof, and
wherein the solid tumor is triple negative breast cancer.
|