| CPC A61K 9/19 (2013.01) [A61K 47/60 (2017.08); A61K 9/127 (2013.01); A61K 9/1277 (2013.01); B82Y 5/00 (2013.01)] | 15 Claims |
|
1. A method of producing a nucleic acid lipid nanoparticle composition, the method comprising:
mixing a lipid solution comprising an ionizable lipid with a solution comprising a nucleic acid thereby forming a precursor nucleic acid lipid nanoparticle, wherein the precursor nucleic acid lipid nanoparticle further comprises a first PEG lipid;
optionally processing the precursor nucleic acid lipid nanoparticle;
adding a second PEG lipid to the precursor nucleic acid lipid nanoparticle thereby forming a modified nucleic acid lipid nanoparticle; and
processing the modified nucleic acid lipid nanoparticle, thereby forming the nucleic acid lipid nanoparticle composition,
wherein:
the first PEG lipid and the second PEG lipid are independently selected from
i) a compound of Formula (PL-II):
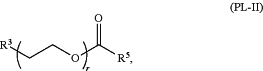 or a salt thereof, wherein:
R3 is —ORO;
RO is hydrogen or unsubstituted alkyl;
r is an integer between 1 and 100;
R5 is optionally substituted C10-40 alkyl, optionally substituted C10-40 alkenyl, or optionally substituted C10-40 alkynyl; and optionally one or more methylene groups of R5 are replaced with optionally substituted carbocyclylene, optionally substituted heterocyclylene, optionally substituted arylene, optionally substituted heteroarylene, N(RN), O, S, C(O), C(O)N(RN), —NRNC(O), NRNC(O)N(RN), C(O)O, OC(O), OC(O)O, OC(O)N(RN), NRNC(O)O, C(O)S, —SC(O), C(═NRN), C(═NRN)N(RN), NRNC(═NRN), NRNC(═NRN)N(RN), C(S), C(S)N(RN), —NRNC(S), NRNC(S)N(RN), S(O), OS(O), S(O)O, OS(O)O, OS(O)2, S(O)2O, OS(O)2O, —N(RN)S(O), S(O)N(RN), N(RN)S(O)N(RN), OS(O)N(RN), N(RN)S(O)O, S(O)2, N(RN)S(O)2, —S(O)2N(RN), N(RN)S(O)2N(RN), OS(O)2N(RN), or N(RN)S(O)2O; and
each instance of RN is independently hydrogen, optionally substituted alkyl, or a nitrogen protecting group, and
ii) a compound of Formula (PL-III):
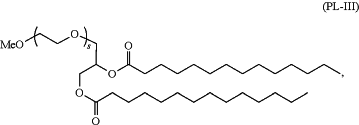 wherein s is an integer between 1 and 100.
|