| CPC A61B 18/1442 (2013.01) [A61B 17/00234 (2013.01); A61B 17/2812 (2013.01); A61B 17/320092 (2013.01); A61B 18/00 (2013.01); A61B 18/04 (2013.01); A61B 18/12 (2013.01); A61B 18/14 (2013.01); A61B 18/1445 (2013.01); A61B 34/25 (2016.02); A61B 46/10 (2016.02); A61B 50/30 (2016.02); A61B 90/40 (2016.02); A61N 7/00 (2013.01); G16H 20/40 (2018.01); G16H 40/63 (2018.01); H01M 10/46 (2013.01); H01M 10/48 (2013.01); H02J 7/0044 (2013.01); H02J 7/0045 (2013.01); H02J 50/10 (2016.02); A61B 17/064 (2013.01); A61B 17/285 (2013.01); A61B 18/1206 (2013.01); A61B 18/1233 (2013.01); A61B 50/33 (2016.02); A61B 90/08 (2016.02); A61B 2017/0046 (2013.01); A61B 2017/00084 (2013.01); A61B 2017/0084 (2013.01); A61B 2017/00398 (2013.01); A61B 2017/00473 (2013.01); A61B 2017/00477 (2013.01); A61B 2017/00482 (2013.01); A61B 2017/00734 (2013.01); A61B 2017/291 (2013.01); A61B 2017/293 (2013.01); A61B 2017/294 (2013.01); A61B 2017/2929 (2013.01); A61B 2017/2931 (2013.01); A61B 2017/2933 (2013.01); A61B 2017/320069 (2017.08); A61B 2017/320071 (2017.08); A61B 2017/320094 (2017.08); A61B 2017/320095 (2017.08); A61B 2018/0019 (2013.01); A61B 2018/00178 (2013.01); A61B 2018/00589 (2013.01); A61B 2018/00595 (2013.01); A61B 2018/00601 (2013.01); A61B 2018/00607 (2013.01); A61B 2018/00791 (2013.01); A61B 2018/00988 (2013.01); A61B 2018/1226 (2013.01); A61B 2018/1412 (2013.01); A61B 2018/1455 (2013.01); A61B 2050/0065 (2016.02); A61B 2050/3008 (2016.02); A61B 2090/0803 (2016.02); A61B 2090/0813 (2016.02); A61B 2090/0814 (2016.02); H01M 2220/30 (2013.01); H02J 7/0048 (2020.01); Y10T 29/49005 (2015.01); Y10T 29/49895 (2015.01); Y10T 29/53913 (2015.01)] | 20 Claims |
|
1. A medical system, comprising:
(a) a medical device, including:
(i) a housing,
(ii) an integral power source positioned within the housing,
(iii) an active element in communication with the integral power source, wherein the active element is operable to perform an operation on a tissue of a patient,
(iv) a recess, and
(v) a first charging feature positioned within the housing in communication with the integral power source;
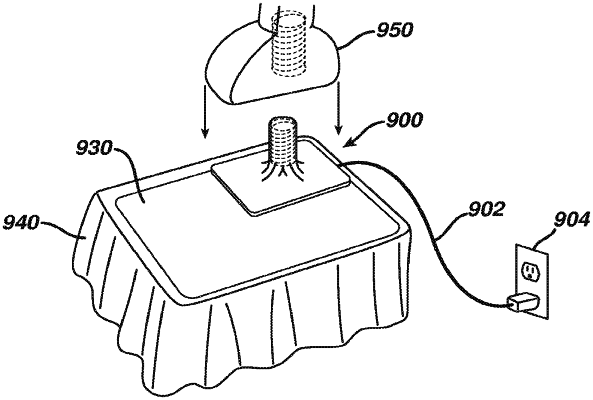
(b) a charging device including a second charging feature, wherein the charging device is configured to communicate with the integral power source of the medical device to thereby charge the integral power source of the medical device when the first charging feature is aligned with the second charging feature, wherein the charging device further includes a base portion and a peg portion extending upwardly from the base portion, wherein the recess of the medical device is configured to receive the peg portion of the charging device such that the peg portion supports the medical device thereon in a predetermined alignment thereby reducing the likelihood of the medical device moving relative to the charging device for supporting the medical device in the predetermined alignment while charging the integral power source of the medical device; and
(c) a sterile barrier having a flexible portion interposed between the first charging feature within the housing and the second charging feature of the charging device, wherein the sterile barrier is configured to permit the second charging feature of the charging device to establish contact with the first charging feature of the medical device without compromising sterility of exterior portions of the medical device.
|