| CPC A01N 1/0284 (2013.01) [A01N 1/0221 (2013.01); A01N 1/0252 (2013.01)] | 20 Claims |
|
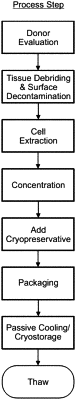
1. A method for processing a biological sample comprising cells, the method comprising:
(a) generating a transplantable unit of the biological sample comprising cells, wherein the transplantable unit comprises a first volume of the cells at a first concentration;
(b) generating a surrogate unit of the biological sample comprising cells, wherein the surrogate unit comprises a second volume of the cells at a second concentration,
wherein the second volume is less than the first volume and the second concentration is no more than 30% different from the first concentration;
(c) cooling the cells of the transplantable unit and the cells of the surrogate unit at a common optimized rate which balances damage from intracellular ice formation with damage from extracellular ice formation; and
(d) storing the transplantable unit and the surrogate unit in the same long-term storage container which exposes the cells of the transplantable unit and the cells of the surrogate unit to similar conditions over a long-term storage duration
thereby providing an equivalent survival and viability for cells from the transplantable unit and for cells from the surrogate unit including a post-thaw cell proliferation rate of the cells of the surrogate unit which is no more than 30% different from a post-thaw cell proliferation rate of the cells of the transplantable unit such that the surrogate unit accurately represents the transplant unit.
|