| CPC G01N 33/4905 (2013.01) [B01L 3/502 (2013.01); B01L 3/561 (2013.01); B01L 3/567 (2013.01); G01N 11/00 (2013.01); G01N 33/86 (2013.01); B01L 2200/0621 (2013.01); B01L 2200/0684 (2013.01); B01L 2200/10 (2013.01); B01L 2300/0627 (2013.01); B01L 2300/087 (2013.01); B01L 2400/049 (2013.01); B01L 2400/0694 (2013.01)] |
| AS A RESULT OF REEXAMINATION, IT HAS BEEN DETERMINED THAT: |
| Claim 10 is cancelled. |
| Claims 1-4, 6-9 and 11-12 are determined to be patentable as amended. |
| Claims 5 and 13, dependent on an amended claim, are determined to be patentable. |
| New claims 14-21 are added and determined to be patentable. |
|
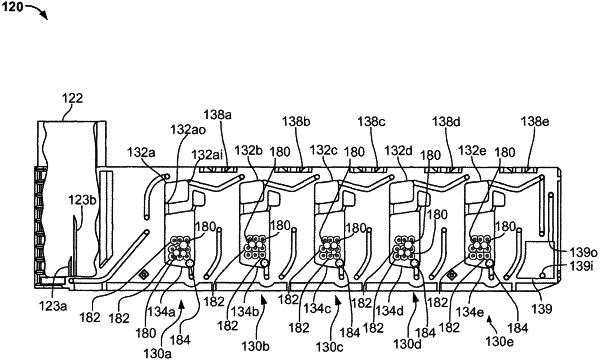
1. A cartridge for use with a blood testing console, the cartridge comprising:
a blood sample receiver configured to receive a blood sample to be tested;
a plurality of blood processing and testing paths arranged in
a blood sample volume measurement chamber in fluid communication with the blood sample receiver, the blood sample volume measurement chamber having a selected internal volume to contain a predefined volume of blood sample from
a mixing chamber in fluid communication with the blood sample volume measurement chamber and with a reagent, the mixing chamber [ being ] configured to receive the predetermined volume of the blood sample from the blood sample volume measurement chamber and [ to ] mix the received blood [ sample ] with the reagent [ to produce a mixture of blood sample and reagent] ;
a viscoelastic blood testing chamber configured to receive
conduits fluidically connecting the blood sample volume measurement chamber, the mixing chamber, and the viscoelastic blood testing chamber, each conduit having a different three-dimensional structure than each of the blood sample volume measurement chamber, the mixing chamber, and the viscoelastic blood testing chamber;
a channel in fluid communication with a blood sample volume measurement chamber of the plurality of blood processing and testing paths, the channel being configured to receive part of the blood sample from the blood sample volume measurement chamber, the channel having a different three-dimensional structure than the blood sample volume measurement chamber, the mixing chamber, and the viscoelastic blood testing chamber; and
a structure configured for use in connection with a sensor in the blood testing console to enable a presence of the part of the blood sample in the channel to be determined] .
|
|
2. The cartridge of claim 1, wherein
|
|
3. The cartridge of claim 2, further comprising a vacuum port at an opposite end of the series of the blood sample volume measurement chambers from the blood sample receiver, wherein the cartridge is configured such that, when an external vacuum is applied to the vacuum port, blood [ sample ] is transported from the blood sample receiver to fill each of the blood sample volume measurement chambers in series.
|
|
4. The cartridge of claim 3, further comprising a first conduit for transporting blood [ sample ] between the blood sample receiver and
|
|
6. The cartridge of claim 1, wherein [ the conduits of ] each of the blood processing and testing paths
|
|
7. The cartridge of claim 5, wherein each of the blood processing and testing paths comprises a first vent to ambient outside of the cartridge, each first vent being positioned such that blood [ sample ] does not flow through the
|
|
8. The cartridge of claim
|
|
9. The cartridge of claim 1, wherein the mixing chamber comprises reagent beads in solid form that
|
|
11. The cartridge of claim
|
|
12. The cartridge of claim 11, further comprising a pressure application port positioned such that, when an outside pressure is applied to the pressure application port and the
|
|
[ 14. A cartridge for use with a blood testing console, the cartridge comprising:
a blood sample receiver configured to receive a blood sample to be tested;
a plurality of blood processing and testing paths arranged in parallel, each blood processing and testing path for receiving a portion of the blood sample, and each blood processing and testing path comprising:
a blood sample volume measurement chamber in fluid communication with the blood sample receiver, the blood sample volume measurement chamber having a selected internal volume to contain a predefined volume of blood sample from a blood sample container;
a mixing chamber in fluid communication with the blood sample volume measurement chamber and with a reagent, the mixing chamber being configured to receive the predetermined volume of the blood sample from the blood sample volume measurement chamber and to mix the received blood sample with the reagent to produce a mixture of blood sample and reagent;
a viscoelastic blood testing chamber configured to receive the mixture of blood sample and reagent from the mixing chamber for a viscoelastic test to be performed on the mixture of blood sample and reagent while the mixture of blood sample and reagent resides in the testing chamber; and
conduits fluidically connecting the blood sample volume measurement chamber, the mixing chamber, and the viscoelastic blood testing chamber, each conduit having a different three-dimensional structure than each of the blood sample volume measurement chamber, the mixing chamber, and the viscoelastic blood testing chamber; and
a port in fluid communication with all of the blood sample volume measurement chambers of the plurality of blood processing and testing paths, the port being configured to have negative pressure applied from a source of negative pressure, the fluid communication between the port and each of the volume measurement chambers including no intervening mixing chamber or viscoelastic blood testing chamber, and the port being different from the blood sample receiver.]
|
|
[ 15. The cartridge of claim 14, wherein the mixing chamber comprises reagent beads in solid form that dissolve when contacted with the blood sample from a corresponding blood sample volume measurement chamber to provide the mixture of blood sample and reagent in the mixing chamber, and wherein the reagent beads comprise reagent compositions including one or more of CaCl2, ellagic acid/phospholipids, tissue factor, heparinase, polybrene, cytochalasin D, or tranexamic acid.]
|
|
[ 16. The cartridge of claim 14, wherein the conduit fluidly connecting the mixing chamber to the corresponding viscoelastic blood testing chamber, in each of the blood processing and testing paths comprises a valve positioned in the conduit, each valve being configured to prevent flow of the mixture of blood sample and reagent through the conduit when in a closed position and to allow the flow of the mixture of blood sample and reagent through the conduit when in an open position.]
|
|
[ 17. The cartridge of claim 16, further comprising a pressure application port positioned such that, when an outside pressure is applied to the pressure application port and the valve is in an open position, the mixture of blood sample and reagent from the mixing chamber is transported through the conduit from the mixing chamber to the corresponding viscoelastic blood testing chamber.]
|
|
[ 18. A cartridge for use with a blood testing console, the cartridge comprising:
a blood sample receiver configured to receive a blood sample to be tested;
a plurality of blood processing and testing paths arranged in parallel, each blood processing and testing path for receiving a portion of the blood sample, and each blood processing and testing path comprising:
a blood sample volume measurement chamber in fluid communication with the blood sample receiver, the blood sample volume measurement chamber having a selected internal volume to contain a predefined volume of blood sample from a blood sample container;
a mixing chamber in fluid communication with the blood sample volume measurement chamber and with a reagent, the mixing chamber being configured to receive the predetermined volume of the blood sample from the blood sample volume measurement chamber and to mix the received blood sample with the reagent to produce a mixture of blood sample and reagent
a viscoelastic blood testing chamber configured to receive the mixture of blood sample and reagent from the mixing chamber for a viscoelastic test to be performed on the mixture of blood sample and reagent while the mixture of blood sample and reagent resides in the testing chamber; and
conduits fluidically connecting the blood sample receiver, the blood sample volume measurement chamber, the mixing chamber, and the viscoelastic blood testing chamber, each conduit having a different three-dimensional structure than each of the blood sample volume measurement chamber, the mixing chamber, and the viscoelastic blood testing chamber;
wherein the blood sample receiver comprises a needle assembly, the needle assembly comprising a needle configured to enter an interior of the blood sample container;
a venting pathway in fluid communication with the needle, the venting pathway being different from the conduits; and
a vent port in fluid communication with the venting pathway, the needle, and an exterior of the cartridge.]
|
|
[ 19. The cartridge of claim 18, wherein the mixing chamber comprises reagent beads in solid form that dissolve when contacted with the blood sample from a corresponding blood sample volume measurement chamber to provide the mixture of blood sample and reagent in the mixing chamber, and wherein the reagent beads comprise reagent compositions including one or more of CaCl2, ellagic acid/phospholipids, tissue factor, heparinase, polybrene, cytochalasin D, or tranexamic acid.]
|
|
[ 20. The cartridge of claim 18, wherein the conduit fluidly connecting the mixing chamber to the corresponding viscoelastic blood testing chamber, in each of the blood processing and testing paths comprises a valve positioned in the conduit, each valve being configured to prevent flow of the mixture of blood sample and reagent through the conduit when in a closed position and to allow the flow of the mixture of blood sample and reagent through the conduit when in an open position.]
|
|
[ 21. The cartridge of claim 20, further comprising a pressure application port positioned such that, when an outside pressure is applied to the pressure application port and the valve is in an open position, the mixture of blood sample and reagent from the mixing chamber is transported through the conduit from the mixing chamber to the corresponding viscoelastic blood testing chamber.]
|