| CPC G01N 35/0099 (2013.01) [B01L 3/50853 (2013.01); B01L 9/523 (2013.01); F24F 11/30 (2018.01); G06K 7/1413 (2013.01); G06Q 10/087 (2013.01); G06Q 10/10 (2013.01); G16H 10/40 (2018.01); G16H 40/40 (2018.01); G16H 40/67 (2018.01); B01L 2200/143 (2013.01); B01L 2300/021 (2013.01); G01N 2035/00356 (2013.01); G05B 2219/40269 (2013.01); H04B 1/3827 (2013.01)] | 33 Claims |
|
1. An assay system configured to use an assay consumable for conducting an assay, said assay consumable associated with a consumable identifier wherein said assay system comprises:
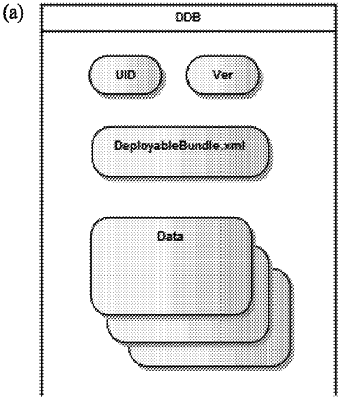
(a) a storage medium configured to store data describing a plurality of steps of a generic assay protocol applicable to a plurality of assays, and configured to store a data deployable bundle (DDB) associated with the consumable identifier, wherein the DDB includes information describing a specific assay protocol to be performed, the specific assay protocol including all or a portion of the plurality of steps of the generic assay protocol;
(b) a controller adapted to:
read the information in the DDB to determine the specific assay protocol to be performed when conducting the assay; and
cause all or a portion of the steps of the generic assay protocol to be performed based on the specific assay protocol associated with the DDB.
|