| CPC C07D 498/04 (2013.01) [A61K 31/4985 (2013.01); A61K 31/506 (2013.01); A61K 31/517 (2013.01); A61K 31/519 (2013.01); A61K 31/5377 (2013.01); A61K 31/553 (2013.01); A61K 31/555 (2013.01); A61K 33/243 (2019.01); A61K 39/3955 (2013.01); A61P 35/00 (2018.01); C07D 487/04 (2013.01)] | 23 Claims |
|
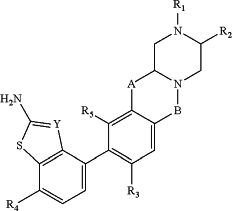
1. A compound of the formula:
 wherein:
A is —OCH2—, —N(R6)CH2—, —OCH2CH2—, —N(R6)CH2CH2—, —CH2OCH2—, or —CH2N(R6)CH2—;
B is —CH2— or —C(O)—,
Y is —C(CN)— or —N—;
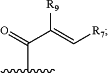
R1 is —CN, —C(O)C≡CR8, or a group of the formula
 R2 is H, methyl, or —CH2CN;
R3 and R5 are each independently H, halogen, —C0-3 alkyl-cyclopropyl, —C1-6 alkyl optionally substituted 1-3 times with R10, or —O—C1-6 alkyl optionally substituted 1-3 times with R10;
R4 is H, halogen, or —C1-6 alkyl optionally substituted 1-3 times with R10;
R6 is H or —C1-6 alkyl optionally substituted 1-3 times with R10;
R7 is H, halogen, —NR11R12, —CH2NR11R12, —C1-6 alkyl optionally substituted 1-3 times with R10 or R13, —C0-3 alkyl cyclopropyl, or —O—C1-6 alkyl optionally substituted 1-3 times with R10 or R13;
R8 is H, —C1-4 alkyl optionally substituted 1-3 times with R10, or —C3-6 cycloalkyl optionally substituted 1-3 times with R10;
R9 is H, halogen, —CN, —C0-3 alkyl-C3-6 cycloalkyl, or —C1-6 alkyl optionally substituted 1-3 times with R10;
R10 is independently at each occurrence halogen, oxygen, hydroxy, —C1-4 alkyl, or —O—C1-4 alkyl;
R11 and R12 are each independently H, —C1-4 alkyl, or —C1-4 heteroalkyl, wherein R11 and R12 may combine to form a cycloheteroalkyl; and
R13 is independently at each occurrence —N—C1-4 alkyl,
or a pharmaceutically acceptable salt thereof.
|