| CPC C07C 2/24 (2013.01) [B01J 31/26 (2013.01); C01C 1/0411 (2013.01); C25B 1/27 (2021.01); C25B 3/03 (2021.01); C25B 9/19 (2021.01); C25B 9/23 (2021.01); C25B 11/042 (2021.01); C25B 11/046 (2021.01); C25B 11/0773 (2021.01); C25B 13/04 (2013.01); C25B 13/07 (2021.01); B01J 2231/20 (2013.01)] | 15 Claims |
|
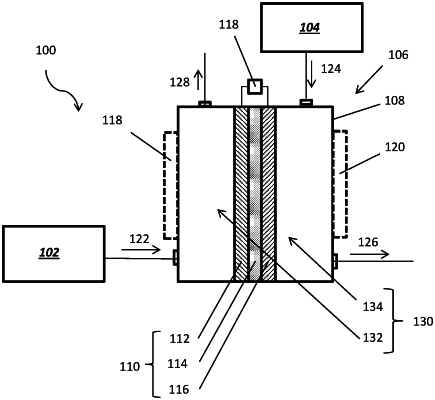
1. A method of forming a hydrocarbon product and ammonia, comprising:
introducing ethane (C2H6) to a positive electrode of an electrochemical cell comprising:
the positive electrode;
a negative electrode comprising one or more of a Ru/perovskite cermet, a RuNi/perovskite cermet, a RuCe/perovskite cermet, and a RuNiCe/perovskite cermet; and
a proton-conducting membrane between the positive electrode and the negative electrode, the proton-conducting membrane comprising an electrolyte material having an ionic conductivity greater than or equal to about 10−2 S/cm at one or more temperatures within a range of from about 150° C. to about 600° C.;
introducing N2 to the negative electrode of the electrochemical cell; and
applying a potential difference between the positive electrode and the negative electrode of the electrochemical cell while the C2H6 interacts with the positive electrode so that hydrogen (H) atoms of the C2H6 release electrons (e−) to produce C2H4, hydrogen ions (H+), and the e− at the positive electrode through non-oxidative deprotonation of the C2H6 at the one or more temperatures, to transport the H+ through the proton-conducting membrane, and to produce NH3 at the negative electrode.
|