| CPC A61K 39/39 (2013.01) [A61K 9/127 (2013.01); A61K 39/0003 (2013.01); A61K 39/008 (2013.01); A61K 39/015 (2013.01); A61K 39/0275 (2013.01); A61K 39/0291 (2013.01); A61K 39/04 (2013.01); A61K 39/05 (2013.01); A61K 39/08 (2013.01); A61K 39/092 (2013.01); A61K 39/095 (2013.01); A61K 39/099 (2013.01); A61K 39/102 (2013.01); A61K 39/107 (2013.01); A61K 39/12 (2013.01); A61K 39/145 (2013.01); A61K 39/165 (2013.01); A61K 39/20 (2013.01); A61K 39/21 (2013.01); A61K 39/245 (2013.01); A61K 39/29 (2013.01); A61K 39/292 (2013.01); A61K 47/02 (2013.01); C07D 487/04 (2013.01); A61K 31/519 (2013.01); A61K 2039/55505 (2013.01); A61K 2039/55511 (2013.01); C07D 403/04 (2013.01)] | 26 Claims |
|
1. A composition comprising an antigen and a compound of Formula (I):
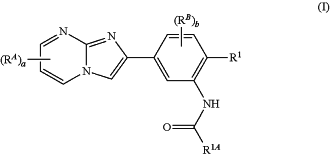 or a pharmaceutically acceptable salt thereof,
wherein:
R1 is hydrogen, halogen, C1-6 alkyl, N(Ra1)2, or ORa;
R1A is benzyl, phenyl, or 6-membered heteroaryl;
wherein the —CH2— portion of the benzyl is optionally substituted with 1 or 2 substituents independently selected from the group consisting of halogen, C1-6 alkyl, N(Ra1)2, and ORa;
wherein each C1-6 alkyl substituent of the —CH2— portion of the benzyl is optionally and independently substituted with 1, 2, or 3 independently selected ORa substituents;
wherein the phenyl portion of the benzyl is optionally substituted with 1, 2, 3, 4, or 5 substituents independently selected from the group consisting of halogen, CN, NO2, alkyl, haloalkyl, alkenyl, alkynyl, C(O)Ra, C(O)N(Ra1)2, C(O)ORa, N(Ra1)2, ORa, SRa, carbocyclyl, heterocyclyl, aryl, and heteroaryl; and
wherein the phenyl or 6-membered heteroaryl is optionally substituted with 1, 2, 3, 4, or 5 substituents independently selected from the group consisting of halogen, CN, NO2, alkyl, haloalkyl, alkenyl, alkynyl, C(O)Ra, C(O)N(Ra1)2, C(O)ORa, N(Ra1)2, ORa, SRa, carbocyclyl, heterocyclyl, aryl, and heteroaryl;
each RA is independently halogen, CN, NO2, alkyl, alkenyl, alkynyl, C(O)Ra, C(O)N(Ra1)2, C(O)ORa, N(Ra1)2, ORa, SRa, carbocyclyl, heterocyclyl, aryl, or heteroaryl;
each RB is independently halogen, CN, NO2, alkyl, alkenyl, alkynyl, C(O)Ra, C(O)N(Ra1)2, C(O)ORa, N(Ra1)2, ORa, SRa, carbocyclyl, heterocyclyl, aryl, or heteroaryl;
each Ra is independently hydrogen, alkyl, alkenyl, alkynyl, acyl, carbocyclyl, heterocyclyl, aryl, or heteroaryl;
each Ra1 is independently hydrogen, alkyl, alkenyl, alkynyl, acyl, carbocyclyl, heterocyclyl, aryl, or heteroaryl; or
any two Ra1, together with the nitrogen atom to which they are attached, independently form a heterocyclyl or heteroaryl;
a is 0, 1, 2, or 3; and
b is 0, 1, 2, or 3.
|