| CPC A61K 31/675 (2013.01) [A61P 1/16 (2018.01); C07F 9/65586 (2013.01); C07F 9/65616 (2013.01)] | 11 Claims |
|
1. A compound according to Formula I or Formula II:
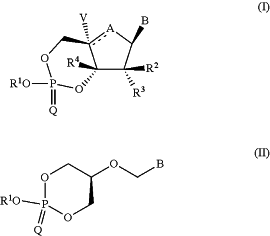 or a pharmaceutically acceptable salt or stereoisomer thereof wherein:
A is selected from O, S, CH2, CF and C═CH2, such that if R2 is OH and R3, R4 and V are hydrogen, then A is other than S; and if A is CF or C═CH2, then V is absent;
B is selected from the following groups:
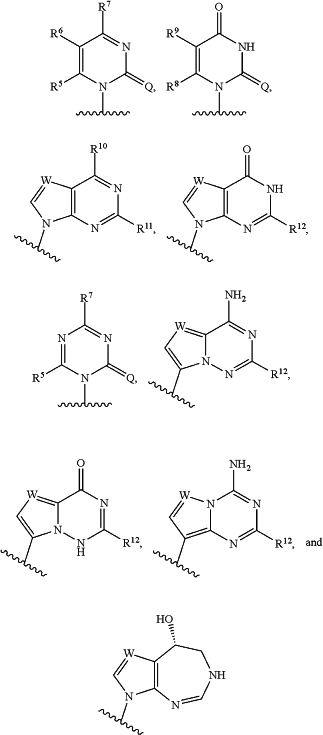 each Q is independently O or S;
V is hydrogen, halogen, —N(R13)2, —OR13, alkyl, alkenyl, alkynyl, haloalkyl, N3 or CN;
W is N, CH or CF;
R1 is —CH2—X—Y—R16;
X is —C(R14)2;
Y is —C(R15)2, or C3-C6 cycloalkylene;
R2 is fluoro, chloro, —OR13, —CN, —N(R13)2, N3, C1-C3alkyl, C1-C3haloalkyl, C2-C6alkenyl or C2-C3alkynyl;
R3 is hydrogen;
R4 is hydrogen, C1-C6alkyl, C2-C6alkenyl, C2-C6alkynyl, C1-C6haloalkyl, —OR13, fluoro, chloro, N3, —CN or —N(R13)2;
R5, R6, R8 and R9 are each independently selected from hydrogen, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, halogen, —OR18, —SR18 and —N(R18)2;
R7, R10, R11 and R12 are each independently selected from hydrogen, C1-C6alkyl, C2-C6 alkenyl, C2-C6alkynyl, C3-C7cycloalkyl, C5-C6heteroaryl, C9-C10heteroaryl, halogen, —OR18, —SR18, —S(O)R18, —S(O)2R18, —S(O)2N(R18)2, —NHC(O)OR18, —NHC(O)N(R18)2, C1-C6haloalkyl, C1-C6hydroxyalkyl, —O(C1-C6haloalkyl), —CN, —NO2, —N(R18)2, —NH(C1-C6alkylene)-(C5-C6heteroaryl), —NH(C1-C6 alkylene)-(C9-C10heteroaryl), —C(O)R18, —C(O)OR18, —C(O)N(R18)2 and —NHC(O)R18, wherein said C2-C6alkenyl group and said C2-C6alkynyl group are optionally substituted with one or more halogen;
each occurrence of R13 is independently selected from hydrogen, C1-C6alkyl —C(O)R18, or —C(O)OR18;
R14 is hydrogen, halogen, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C6-C10aryl-, OR17, —OC(O)R17, —N(R12)C(O)OR17 or —C(O)OR17;
each occurrence of R15 is independently selected from hydrogen, halogen, C1-C6alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, C6-C10aryl-, OR17, —OC(O)R17, —N(R12)C(O)OR17 and —C(O)OR17 or both R15 groups together with the carbon atom to which they are attached can join to form a 3- to 6-membered spirocyclic cycloalkyl group, such that at least one R15 group cannot be hydrogen;
R16 is —C(O)OR17;
each occurrence of R17 is independently selected from hydrogen, halogen, C1-C6alkyl, C7cycloalkyl and C6-C10aryl;
each occurrence of R18 is independently selected from hydrogen, C1-C15alkyl, C1-C6haloalkyl, C1-C6hydroxyalkyl, —(C1-C3alkylene)m-(C3-C7cycloalkyl), —(C1-C3alkylene)m-(C6-C10aryl), —(C1-C3alkylene)m-(C4-C7heterocycloalkyl), —(C1-C3alkylene)m-(C5-C6heteroaryl) and —(C1-C3alkylene)m-(C9-C10heteroaryl); and
each occurrence of m is independently 0 or 1.
|