| CPC G16H 50/50 (2018.01) [G16H 20/30 (2018.01); G16H 40/67 (2018.01); G16H 50/70 (2018.01); G16H 70/60 (2018.01); A61N 1/36114 (2013.01)] | 13 Claims |
|
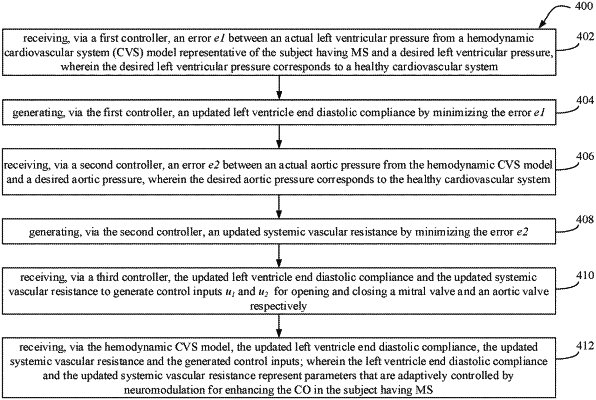
1. A processor implemented method (400) for enhancing cardiac output (CO) in a subject having Mitral Stenosis (MS), the method comprising the steps of:
receiving, via one or more hardware processors serving as a first controller, an error e1 between an actual left ventricular pressure Plv obtained from an adaptive controller comprising a hemodynamic cardiovascular system (CVS) model representative of the subject having MS and a desired left ventricular pressure pa wherein the desired left ventricular pressure corresponds to a healthy cardiovascular system (402);
generating an updated left ventricle end diastolic compliance clv,d via the first controller, by minimizing the error e1 (404);
receiving, via one or more hardware processors serving as a second controller, an error e2 between an actual aortic pressure Psa obtained from an adaptive controller comprising the hemodynamic CVS model and a desired aortic pressure Psad, wherein the desired aortic pressure corresponds to the healthy cardiovascular system (406);
generating an updated systemic vascular resistance Rs, via the second controller, by minimizing the error e2 (408);
receiving, via one or more hardware processors serving as a third controller, the updated left ventricle end diastolic compliance clv,d and the updated systemic vascular resistance Rs to generate control inputs u1 and u2 for opening and closing a mitral valve and an aortic valve respectively (410) of the subject;
receiving, via the adaptive controller comprising the hemodynamic CVS model, the updated left ventricle end diastolic compliance clv,d, the updated systemic vascular resistance Rs and the generated control inputs u1 and u2 (412); and
enhancing, via the one or more hardware processors serving as the first controller, the second controller and the third controller, the CO in the subject having MS by using the generated control inputs u1 and u2 and adaptively controlling via the hemodynamic CVS model, the parameters of updated left ventricle end diastolic compliance clv,d, and the updated systemic vascular resistance Rs by neuromodulation.
|