| CPC G16H 40/20 (2018.01) [G16H 10/40 (2018.01); G16H 40/40 (2018.01)] | 17 Claims |
|
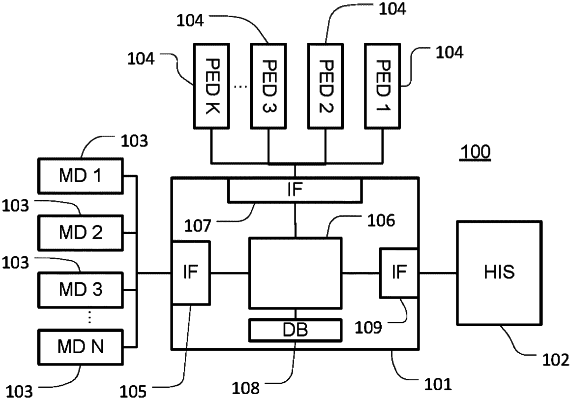
1. A system of medical devices, the system comprising:
a medical device data management system comprising a processor;
a plurality of medical devices communicatively connected to the medical device data management system; each of the medical devices being operable to analyze one or more samples of biological material; and to communicate information about an operational state of the medical device to the medical device data management system; and
a plurality of portable electronic devices, each of the portable electronic devices operable to be carried by an operator, each of the portable electronic devices communicatively connectable to the medical device data management system and configured to receive, from the medical device data management system, information indicative of an operational state of each of the medical devices and to display the received information in respect of one or more selected devices of the medical devices,
wherein:
the information about an operational state received by the portable electronic device from the medical device data management system includes an availability prediction;
one or more of the portable electronic devices are configured to transmit, to the medical device data management system, an indication of an intended measurement to be taken on a biological sample by an operator associated with the portable electronic device; and wherein the medical device data management system is configured to determine the availability prediction based at least in part on the information received from the medical devices and on the indications received from the one or more portable electronic devices; and
the medical device data management system is configured to:
obtain device location information about respective locations of at least a subset of the medical devices and operator location information about a location of said operator; and
determine the availability prediction at least in part on the information received from the medical devices, on the indications received from the one or more portable electronic devices and on the obtained device location information and operator location information.
|