| CPC A61M 5/172 (2013.01) [A61M 60/113 (2021.01); A61M 60/148 (2021.01); A61M 60/178 (2021.01); A61M 60/232 (2021.01); A61M 60/237 (2021.01); A61M 60/279 (2021.01); A61M 60/30 (2021.01); A61M 60/422 (2021.01); A61M 60/515 (2021.01); A61M 60/538 (2021.01); A61M 60/585 (2021.01); A61M 60/857 (2021.01); A61M 2205/18 (2013.01); A61M 2205/3303 (2013.01); A61M 2205/3317 (2013.01); A61M 2205/3375 (2013.01); A61M 2205/3507 (2013.01); A61M 2205/3553 (2013.01); A61M 2205/3584 (2013.01); A61M 2205/50 (2013.01); A61M 2205/502 (2013.01)] | 22 Claims |
|
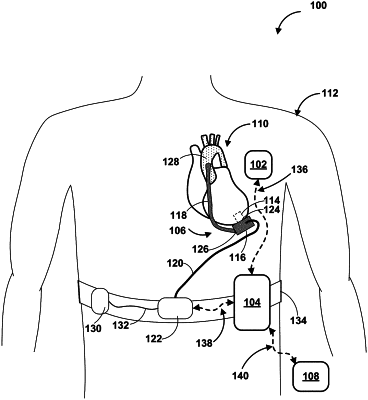
1. An implantable medical device comprising:
communication circuitry;
a transducer configured to generate a signal representative of a mechanical wave from a mechanical circulatory support device separate from the implantable medical device; and
processing circuitry communicatively coupled to the transducer, wherein the processing circuitry is configured to:
determine an indication of pump thrombosis based on the signal and,
based on the indication of pump thrombosis, communicate the indication of pump thrombosis to an external user interface device via the communication circuitry.
|
|
12. A method comprising:
receiving, by processing circuitry of an implantable medical device, a signal from a transducer of the implantable medical device representative of a mechanical wave generated by a mechanical circulatory support device separate from the implantable medical device;
determining, by the processing circuitry, an indication of pump thrombosis based on the signal; and
based on the indication of the pump thrombosis, communicating, by the processing circuitry, the indication of pump thrombosis to an external user interface device.
|