| CPC G01N 33/564 (2013.01) [A61K 31/192 (2013.01); A61K 31/216 (2013.01); A61K 31/575 (2013.01); A61P 1/16 (2018.01); G01N 33/6863 (2013.01); G01N 2333/521 (2013.01); G01N 2800/08 (2013.01); G01N 2800/085 (2013.01); G01N 2800/24 (2013.01); G01N 2800/52 (2013.01)] | 13 Claims |
|
1. A method for treating a subject diagnosed with an autoimmune hepatitis (AIH), the method comprising:
administering to a subject an effective amount of:
an immunosuppressive agent,
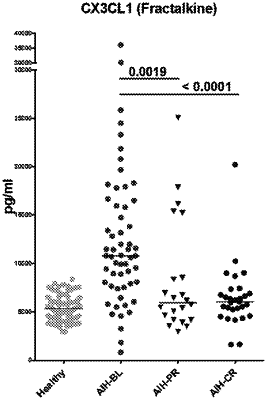
wherein the subject is identified as in need for treatment for the AIH based on having, in a body fluid sample, a level of CX3CL1 higher than a threshold level of CX3CL1.
|