| CPC C07D 471/04 (2013.01) [A61K 31/416 (2013.01); A61K 31/437 (2013.01); A61P 11/00 (2018.01); A61P 11/06 (2018.01); A61P 43/00 (2018.01)] | 7 Claims |
|
1. A method of treating a respiratory disease modulated by Janus kinases in a subject having said disease, the method comprising administering to the subject a pharmaceutical composition comprising a compound of formula (I):
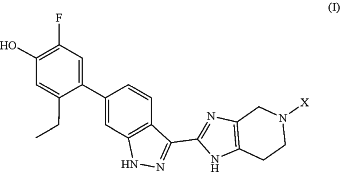 wherein:
X is a group of formula (II):
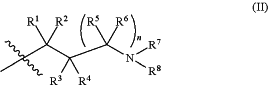 n is 0 or 1;
R1 is hydrogen or C1-3alkyl;
R2 is hydrogen or C1-3alkyl;
R3 is hydrogen or C1-3alkyl;
or R2 and R3 taken together form C2-4alkylene;
or, when n is 1, R3 is selected from hydrogen, —OH, —OC1-3alkyl, halo, —C(O)OC1-3alkyl, and C1-3alkyl, wherein C1-3alkyl is optionally substituted with —OH;
R4 is hydrogen or C1-3alkyl;
R5 is selected from hydrogen, C1-3alkyl, —C(O)OC1-3alkyl, and phenyl;
or when n is 1, R2 and R5 taken together form C1-3alkylene;
R6 is hydrogen or C1-3alkyl;
R7 is hydrogen or C1-3alkyl,
or when n is 0, R2 and R7 taken together form C1-3alkylene, or
R4 and R7 taken together form C2-4alkylene or C1alkylene-O—C2alkylene;
or when n is 1, R2 and R7 taken together form C2alkylene, optionally substituted with C1-3alkyl or Rx,
or R4 and R7 taken together form C1-3alkylene or —O—C2alkylene;
R8 is selected from
(a) hydrogen,
(b) methyl, optionally substituted with —CN, phenyl or C3-6cycloalkyl;
(c) C2-6alkyl, wherein C2-6alkyl is optionally substituted with one or two substituents selected from —OH, —OC1-3alkyl, —CN, —SC1-3alkyl, phenyl, C3-6cycloalkyl, halo, and optionally, in addition with two substituents on a single carbon atom taken together to form C2-3alkylene;
(d) C3-6cycloalkyl, wherein C3-6cycloalkyl is optionally substituted with —OH, —CN, —OC1-3alkyl, or C1-3alkyl, wherein C1-3alkyl is optionally substituted with —OC1-3alkyl or with one or two halo,
(e) oxetanyl,
(f) tetrahydropyranyl,
(g) tetrahydrothiophenyl 1,1-dioxide, and
(h) phenyl,
or R7 and R8 taken together form C3-5alkylene or C2alkylene-O—C2alkylene;
wherein C3-5alkylene is optionally substituted with one or two Rx;
Rx is selected from —OH, —CN, —OC1-3alkyl, halo, phenyl, and C1-3alkyl which is optionally substituted with —OC1-3alkyl or —OH, or
two substituents Rx taken together form C1-5alkylene or —CH2OCH2—,
or when n is 1 and R2 and R7 taken together form C2alkylene, R4 and a substituent Rx on C2alkylene taken together form C2alkylene;
provided that two substituents Rx on the same carbon atom are not both fluoro, and
provided that when Rx is attached to a carbon atom adjacent to a nitrogen atom, Rx is not —OH, —OC1-3alkyl, or halo;
or a pharmaceutically acceptable salt thereof,
and a pharmaceutically acceptable carrier;
wherein the pharmaceutical composition is a nebulizer composition.
|