| CPC C07D 413/12 (2013.01) [A61P 3/10 (2018.01); A61P 27/02 (2018.01); C07D 403/14 (2013.01); C07D 405/14 (2013.01); C07D 413/14 (2013.01); C07D 417/12 (2013.01)] | 19 Claims |
|
1. A compound of the general formula
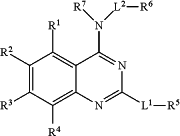 or a salt, hydrate, solvate, tautomer or stereoisomer thereof,
wherein:
R1 is selected from the group consisting of H, hydroxyl, halo, C1-6 alkyl, C1-4 alkoxy, O-CH2phenyl, and O-phenyl;
R2 is selected from the group consisting of H, hydroxyl, halo, C1-6 alkyl, C1-4 alkoxy, O-CH2phenyl, and O-phenyl;
R3 is selected from the group consisting of H, hydroxyl, halo, C1-6 alkyl, C1-4 alkoxy, O-CH2phenyl, and O-phenyl;
R4 is selected from the group consisting of H, hydroxyl, halo, C1-6 alkyl, C1-4 alkoxy, O-CH2phenyl, and O-phenyl;
or R1 and R2, or R2 and R3, or R3 and R4 together form C1-3alkylenedioxy;
wherein at least one of R1, R2, R3 and R4 is not H,
L1 is selected from the group consisting of NHC1-4alkyl-NHC(O)—, azetidinyl-NHC(O)—, and azetidinyl-NHSO2—;
R5 is selected from the group consisting of C3-9 cycloalkyl, naphthyl, C2-9 N-heteroaryl optionally substituted with 1 or 2 RX groups, morpholinyl, piperidinyl, piperazinyl, and C1-4 alkylC2-5 heterocycloalkyl optionally substituted with 1 or 2 RX groups;
L2 is selected from the group consisting of C1-4 alkyl, azetidinyl-C(O)—, C1-4alkyl-NHC(O)—, C1-4alkyl-NHSO2—, —C(O)—, and —SO2—; or L2 is absent;
R6 is selected from the group consisting of H, C2-6 alkyl, guanidinyl, NHC(NH)NH(C1-3alkyl), ureido, NHC(O)NH(C1-3alkyl), C6-10 aryl optionally substituted with 1 or 2 RX groups, C1-9 heteroaryl optionally substituted with 1 or 2 RX groups, C2-5 heterocycloalkyl optionally substituted with 1 or 2 RX groups, and C3-9cycloalkyl optionally substituted with 1 or 2 RX groups;
R7 is H or C1-6 alkyl;
each RX is independently selected from the group consisting of hydroxyl, halo, nitro, NR′R″ (wherein R′ and R″ are independently selected from H and C1-3alkyl), C1-4 alkyl, C3-9cycloalkyl, haloC1-4alkyl, C1-4alkoxy, C(O)C1-3alkyl, C(O)OC1-4alkyl, C(O)NHRY, C6-10aryl optionally substituted with 1 or 2 RY groups, C2-9heteroaryl optionally substituted with 1 or 2 RY groups, C1-4alkyl-(C2-9heteroaryl), C2-5heterocycloalkyl optionally substituted with 1 or 2 C1-4alkyl groups, C1-4alkyl-(C2-5heterocycloalkyl) optionally substituted with 1 or 2 C1-4alkyl groups, C(O)—C2-9heteroaryl optionally substituted with 1 or 2 C1-4 alkyl groups, SO2-C2-9heteroaryl optionally substituted with 1 or 2 C1-4 alkyl groups, and haloC1-4 alkyl groups;
or two adjacent RX groups together form C1-3alkylenedioxy; and
RY is selected from the group consisting of H, hydroxyl, halo, C1-4alkyl, haloC1-4alkyl, and C1-4alkoxy.
|