| CPC C07D 401/14 (2013.01) [C07D 471/04 (2013.01); C07D 471/08 (2013.01); C07D 491/048 (2013.01); C07D 491/107 (2013.01); C07D 491/113 (2013.01); C07D 498/08 (2013.01); C07D 519/00 (2013.01); C07B 2200/05 (2013.01); C07B 2200/07 (2013.01)] | 8 Claims |
|
1. A compound of Formula (II-A):
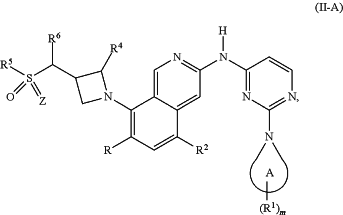 or a pharmaceutically acceptable salt thereof, wherein:
Z is O;
R is H, halogen, or CH3;
Ring A is piperidinyl optionally substituted with 1-6 R1;
each R1 is independently halogen, CN, OH, NRaRb, C1-C4 alkyl, C1-C4 alkoxy, C3-C6 cycloalkyl or —O-C3-C6 cycloalkyl, wherein the alkyl, alkoxy or cycloalkyl represented by R1 or in the group represented by R1 is optionally substituted with 1 to 3 groups selected from deuterium, halogen, OH, NRaRb, C1-C2 alkyl, and C1-C2 alkoxy;
m is 0, 1, 2, 3, 4, 5, or 6;
R2 is H or isopropyl;
R4 is H or methyl;
R5 is H; C1-C4 alkyl optionally substituted with 1 to 3 three groups selected from halogen, CN, and NRaRb; C3-C6 cycloalkyl; or 4-6 membered monocyclic heterocyclyl optionally substituted with C1-C4 alkyl;
R6 is H, CH3, or CH2NH2; and
wherein Ra and Rb are each independently selected from H, methyl and ethyl.
|