| CPC C07D 233/78 (2013.01) [A61K 31/496 (2013.01); A61K 31/5377 (2013.01); A61K 31/55 (2013.01); A61P 19/02 (2018.01); A61P 19/04 (2018.01); C07D 401/04 (2013.01); C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 403/06 (2013.01); C07D 403/14 (2013.01); C07D 405/12 (2013.01); C07D 417/12 (2013.01)] | 16 Claims |
|
1. A compound of formula (II):
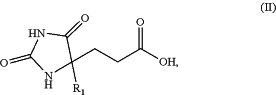 wherein:
R1 is:
C2-4 alkyl optionally substituted with one or more independently selected R4 groups,
C3-7 monocyclic cycloalkyl optionally substituted with one or more independently selected R4 groups,
4-7 membered monocyclic heterocycloalkyl comprising 1 to 2 heteroatoms independently selected from N, O, and S, optionally substituted with one or more independently selected C1-4 alkyl, —C(═O)C1-4 alkyl, or —C(═O)OC1-4 alkyl,
phenyl fused to a 5-6 membered monocyclic heterocycloalkyl comprising 1, 2 or 3 heteroatoms independently selected from N, O, and S, which heterocycloalkyl is optionally substituted with one or more ═O, or
imidazolyl, pyrazolyl, oxazolyl, pyridinyl, pyrimidinyl or pyrazinyl, each of which is optionally substituted with one or more independently selected R5 groups;
R4 is:
halo,
OH,
CN,
C1-4 alkyl,
C1-4 alkoxy optionally substituted with one C1-4 alkoxy, or phenyl,
C1-4 thioalkoxy,
4-7-membered monocyclic heterocycloalkyl comprising one or more heteroatoms independently selected from N, S, and O, optionally substituted with one or more independently selected halo, or —C(═O)OC1-4 alkyl,
phenyl,
—S(═O)2C1-4 alkyl,
—C(═O)NR7bR7c,
—NHC(═O)OR7d,
—NHC(═O)R7e, or
NR8aR8b;
each R5 is
halo,
OH,
CN,
C1-4 alkyl optionally substituted with one or more independently selected halo, —NR9aR9b, or —C(═O)NR9cR9d,
C1-4 alkoxy optionally substituted with one —NR9eR9f, or
—S(═O)2C1-4 alkyl;
each R6 is
halo,
—CN,
—NO2,
—CH3,
5-10 membered monocyclic or fused bicyclic heteroaryl comprising 1, 2 or 3 heteroatoms independently selected from N, O, and S, optionally substituted with one or more independently selected halo, C1-4 alkyl, or C1-4 alkoxy, or
—NR9gR9h;
each R7a, R7b, R7c, R7d, or R7e is
H, or
C1-4 alkyl optionally substituted with one OH, C1-4 alkoxy;
each R8a, or R8b is independently selected from
H, and
C1-4 alkyl optionally substituted with one or more independently selected OH, C1-4 alkoxy, or phenyl;
each R9a, R9b, R9c, R9d, R9e, R9f, R9g, and R9h is independently selected from H, and C1-4 alkyl; or
a pharmaceutically acceptable salt, or a solvate, or a pharmaceutically acceptable salt of a solvate thereof.
|
|
6. A method of making a compound of formula (III),
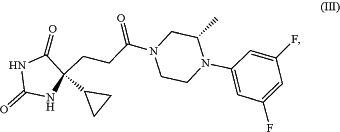 comprising reacting compound (163),
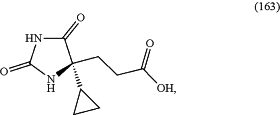 with (2S)-1-(3,5-difluorophenyl)-2-methyl-piperazine.
|