| CPC C07D 233/46 (2013.01) [A61K 31/4178 (2013.01); A61K 45/06 (2013.01); A61P 31/04 (2018.01); C07D 403/12 (2013.01); A61K 38/12 (2013.01)] | 17 Claims |
|
1. A method of preparing a compound of Formula (I)
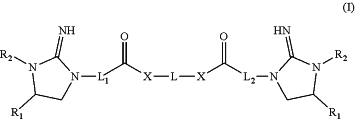 wherein
R1 at each occurrence is independently hydrogen, C1-C10 alkyl, alkenyl, alkynyl, aryl, aryl-C1-C6 alkyl, cycloalkyl, or cycloalkyl-C1-C6 alkyl;
R2 at each occurrence is independently hydrogen, C1-C10 alkyl, alkenyl, alkynyl, aryl, aryl-C1-C6 alkyl, cycloalkyl, or cycloalkyl-C1-C6 alkyl;
X is O or NH
L1 is —(CRxRy)n1;
L2 is —(CRxRy)n2;
L is —(CRxRy)n3—(CH2CH2O)m1-G-(CH2CH2O)m2—(CRxRy)n4—
Rx and Ry at each occurrence is independently hydrogen or C1-C4 alkyl;
G is a bond or -(G1)t-, wherein G1 at each occurrence is independently aryl, cycloakyl, heteroaryl, or heterocycle;
n1 and n2 are each independently 1-4;
m1, m2, n3, and n4 are each independently 0-10;
t is 1, 2, 3, or 4; and
wherein R1, R2, Rx, Ry, and G1 optionally are each independently substituted with one or more substituents selected from the group consisting of halogen, cyano, —OH, C1-C6 alkoxy, —COOH, C1-C6 alkoxycarbonyl, oxo, and amino;
comprising:
reacting an intermediate of Formula (I-i)
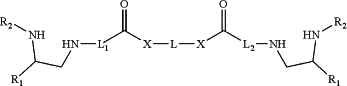 to form the compound of Formula (I).
|