| CPC C07D 231/10 (2013.01) [C07C 227/18 (2013.01)] | 19 Claims |
|
1. A process for the synthesis of a compound of formula (I),
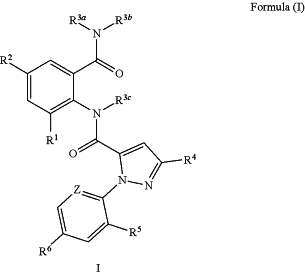 wherein,
R1 is C1-C4 alkyl or halogen;
R2 is hydrogen, halogen or cyano;
R3a and R3b are independently hydrogen, C1-C4 alkyl or C3-C6 cycloalkyl-C1-C4 alkyl;
R3c are independently hydrogen or C1-C4 alkyl;
R4 is halogen, CF3, OCF2H, OCH2CF3, or
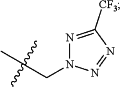 R5 is halogen;
R6 is hydrogen, halogen;
Z is CR7 or N; and
R7 is hydrogen or halogen,
comprising the steps of:
a) reacting a N-substituted anthranilic amide compound of formula (VI) with a pyrazole acid compound of formula (VII) to obtain the compound of formula (I),
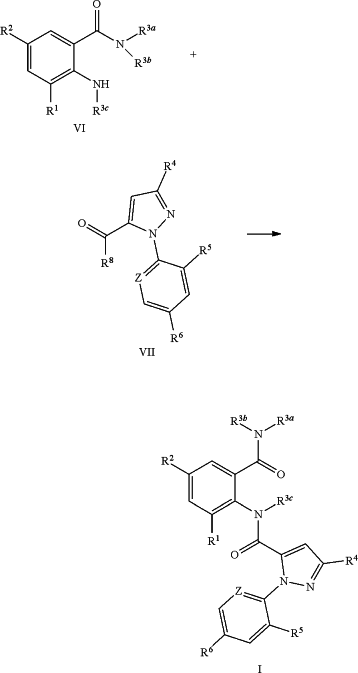 wherein, R8 is OH, Cl, or O—C1-C4 alkyl;
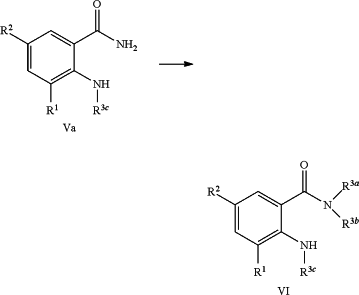
b) converting an anthranilic amide compound of formula (Va) into the N-substituted anthranilic amide compound of formula (VI) by either of the following steps:
i. in the presence of a suitable base or a suitable acid and a suitable alkylating reagent,
ii. by using suitable transamidation process,
according to the reaction scheme as depicted below:
 or
converting an anthranilic acid compound of formula (V) into the N-substituted anthranilic amide compound of formula (VI) using a suitable amine of formula HN(R3a)(R3b) and a suitable coupling agent according to the reaction scheme as depicted below:
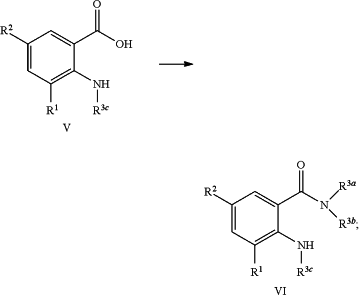 c) converting a mono- or dicyano substituted aniline compound of formula (IV) into the anthranilic acid compound of formula (V) or the anthranilic amide compound of formula (Va) optionally in the presence of a suitable base or a suitable acid according to the reaction scheme as depicted below:
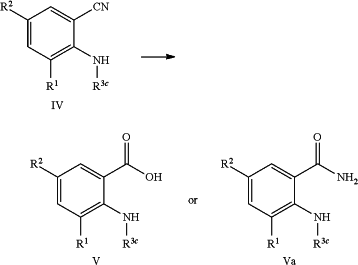 or
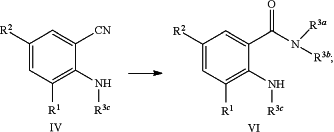
converting a mono- or dicyano substituted aniline compound of formula (IV) into the N-substituted anthranilic amide compound of formula (VI) by either of the following reaction steps:
i) in the presence of a suitable base or a suitable acid and a suitable alkylating reagent,
ii) aminolysis using a suitable amine of formula HN(R3a)(R3b) according to the reaction scheme as depicted below:
 d) optionally, halogenating a mono- or dicyano substituted aniline compound of formula (IV), wherein R1 and/or R2 are hydrogen in the presence of a suitable halogenating agent to obtain a mono- or dicyano substituted aniline compound of formula (IV), wherein R1 and/or R2 are halogen;
e) converting a mono-, di-, or tri-halogenated aniline compound of formula (III) wherein X is halogen, into the mono- or dicyano substituted aniline compound of formula (IV) using a suitable cyanation reagent according to the reaction scheme as depicted below:
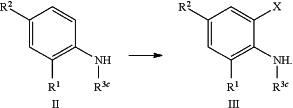
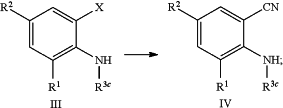 f) converting a substituted aniline compound of formula (II) into the mono-, di-, or tri-halogenated aniline compound of formula (III) wherein X is halogen, using a suitable halogenating agent, according to the reaction scheme as depicted below:
 |