| CPC C07D 211/54 (2013.01) [C07C 255/42 (2013.01); C07D 209/82 (2013.01); C07D 213/38 (2013.01); C07D 213/74 (2013.01); C07D 215/44 (2013.01); C07D 239/42 (2013.01); C07D 307/91 (2013.01); C07D 333/76 (2013.01); C07D 401/12 (2013.01); C07D 405/12 (2013.01); C07D 409/12 (2013.01); C07D 471/04 (2013.01); C07F 7/0812 (2013.01); H10K 30/00 (2023.02); H10K 50/15 (2023.02); H10K 85/40 (2023.02); H10K 85/615 (2023.02); H10K 85/631 (2023.02); H10K 85/633 (2023.02); H10K 85/636 (2023.02); H10K 85/652 (2023.02); H10K 85/654 (2023.02); H10K 85/6572 (2023.02); H10K 85/6576 (2023.02); C07B 2200/05 (2013.01)] | 11 Claims |
|
1. A nitrogen-containing compound, having a structure represented by Formula 1:
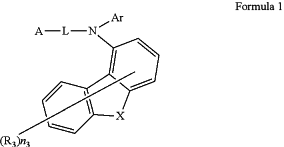 wherein X is selected from O, S, N(R4), or C(R5R6), R4 is selected from an aryl with 6 to 12 carbon atoms, or a heteroaryl with 3 to 12 carbon atoms; R5 to R6 are each independently selected from an alkyl with 1 to 4 carbon atoms, or an aryl with 6 to 12 carbon atoms;
Ar is selected from a substituted or unsubstituted aryl with 6 to 26 carbon atoms, or a substituted or unsubstituted heteroaryl with 3 to 24 carbon atoms;
L is selected from single bond, or a substituted or unsubstituted arylene with 6 to 20 carbon atoms;
A has a structure represented by the following Formula 1-1 or Formula 1-2:
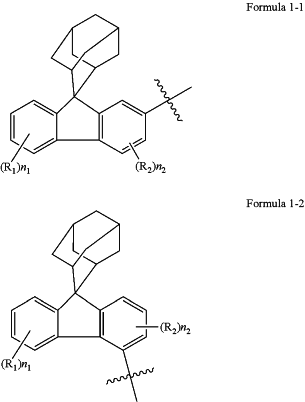 the substituents in L and Ar are the same or different, and are each independently selected from deuterium, fluorine, cyano, an alkyl with 1 to 4 carbon atoms, an alkoxy with 1 to 4 carbon atoms, an alkylthio with 1 to 4 carbon atoms, a haloalkyl with 1 to 4 carbon atoms, a cycloalkyl with 5 to 10 carbon atoms, an aryl with 6 to 15 carbon atoms, a heteroaryl with 5 to 12 carbon atoms, a trialkylsilyl with 3 to 7 carbon atoms, or triphenylsilyl; in L and Ar, optionally, any two adjacent substituents form a ring;
R1 to R3 are the same or different, and are each independently selected from deuterium, fluorine, cyano, an alkyl with 1 to 4 carbon atoms, an alkoxy with 1 to 4 carbon atoms, an alkylthio with 1 to 4 carbon atoms, a fluoroalkyl with 1 to 4 carbon atoms, a cycloalkyl with 5 to 10 carbon atoms, a trialkylsilyl with 3 to 7 carbon atoms, or an aryl with 6 to 12 carbon atoms; and
n1, n2 and n3 respectively represent the number of R1, R2 and R3; R1 to R3 are represented by Rj, and n1 to n3 are represented by nj, wherein j is a variable representing an integer of 1 to 3; and when j is 1, nj is selected from 0, 1, 2, 3, or 4; when j is 2, nj is selected from 0, 1, 2, or 3; when j is 3, nj is 0, 1, 2, 3, 4, 5, 6, or 7; optionally, any two adjacent Rj form a ring.
|