| CPC C07C 209/80 (2013.01) [C07C 209/86 (2013.01)] | 19 Claims |
|
1. A method for synthesizing a compound of formula
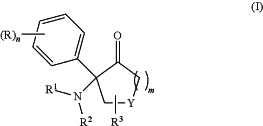 wherein each R independently represents an optionally substituted aryl, heteroaryl, alkyl, perfluoroalkyl, cycloalkyl, alkoxy, aryloxy, acyl, carboxyl, hydroxyl, halogen, amino, nitro, cyano, sulfo or sulfhydryl group, in ortho, meta or para position to the cycloalkylamine moiety;
R1 and R2 each independently represents a hydrogen atom, a lower alkyl group or a cycloalkyl group;
R3 represents a hydrogen group, substituted aryl, heteroaryl, alkyl, perfluoroalkyl, cycloalkyl, alkoxy, aryloxy group;
Y represents an oxygen atom, a sulfur atom, a NH group, a NR4 group or a CH2 group;
R4 represents a hydrogen atom or an alkyl, aryl or a heteroaryl group;
n and m each independently represents an integer from 1 to 5;
or a pharmaceutically acceptable salt thereof; or a precursor thereof; wherein the method comprises step (a) of reacting a compound of formula (II)
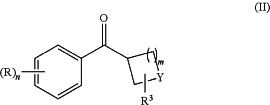 wherein R, R3, Y, n and m are as defined above in relation to the compound of formula (I) with an oxygenating agent, a first additive, and a second additive in a solvent in a fluidic network under thermal and/or photochemical conditions to form a compound of formula (III)
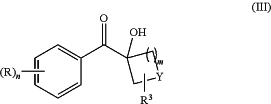 wherein R, R3, Y, n and m are as defined above in relation to the compound of formula (I); and optionally step
(b) of reacting a compound of formula (III) with a nitrogen containing nucleophile in the presence of a third additive and/or a solvent in the fluidic network or in a batch process under thermal conditions to form a compound of formula (IV)
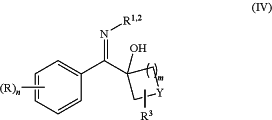 wherein R, R1, R2, R3, Y, n and m are as defined above in relation to the compound of formula (I); and optionally
(c) reacting a compound of formula (IV) in a fluidic network, optionally in the presence of a fourth additive, under thermal conditions to form a compound of formula (I);
wherein a fluidic network comprises one or more micro- and/or meso-channels having an internal dimension of from 100 μm to 2000 μm; wherein a lower alkyl group is a saturated optionally substituted straight- or branched-chain hydrocarbon containing from 1 to 4 carbon atoms; wherein the oxygenating agent is gaseous dioxygen, a solvent saturated in dioxygen, air, or a peroxide.
|