| CPC A61N 1/37512 (2017.08) [A61N 1/0595 (2013.01); A61N 1/37217 (2013.01); G16H 40/67 (2018.01)] | 20 Claims |
|
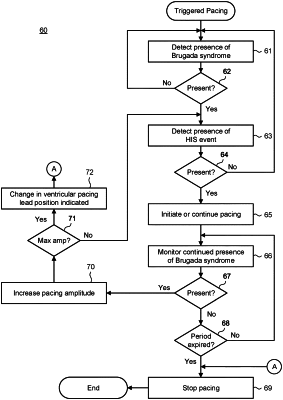
1. A system for Brugada syndrome presence-based electrical therapeutic stimulation delivery comprising:
a cardiac pacing device comprising a pulse generator and a pair of pacing electrodes electrically coupled to the pulse generator via an endocardial lead and positioned in one of a region near the His bundle and a para-Hisian region of a patient's heart, the pulse generator configured to deliver electrical therapeutic stimulation through the pair of pacing electrodes substantially coincident to propagation of an activation wave front proceeding from the atrioventricular node of the patient's heart; and
a diagnostic module operatively coupled to the pulse generator and configured to sense via one or more sensing electrodes physiology associated with a presence of Brugada syndrome in the patient, the diagnostic module further configured to control the pulse generator in delivering the electrical therapeutic stimulation in response to the presence of the Brugada syndrome, wherein the physiology associated with the presence of Brugada syndrome comprises a QRS duration in a lead V2 longer than 90 msec and one of an inferolateral J wave and a horizontal ST segment morphology following a J wave.
|