| CPC A61M 15/0085 (2013.01) [A24B 15/167 (2016.11); A24F 40/05 (2020.01); A24F 40/10 (2020.01); A24F 40/44 (2020.01); A24F 40/48 (2020.01); A24F 40/50 (2020.01); A24F 40/51 (2020.01); A24F 40/53 (2020.01); A24F 40/65 (2020.01); A61M 11/005 (2013.01); A61M 15/06 (2013.01); B05B 17/063 (2013.01); B05B 17/0661 (2013.01); B05B 17/0669 (2013.01); B05B 17/0684 (2013.01); A61M 15/0021 (2014.02); A61M 15/0081 (2014.02); A61M 2205/0294 (2013.01); A61M 2205/276 (2013.01); A61M 2205/3331 (2013.01); A61M 2205/3592 (2013.01); A61M 2205/50 (2013.01); A61M 2205/52 (2013.01); A61M 2205/60 (2013.01); A61M 2205/8206 (2013.01); A61M 2205/8212 (2013.01); A61M 2205/8237 (2013.01); A61M 2230/40 (2013.01); B05B 17/0646 (2013.01); B06B 2201/77 (2013.01)] | 19 Claims |
|
1. A mist inhaler device for generating a mist for inhalation by a user, the mist inhaler device comprising:
a mist generator device comprising:
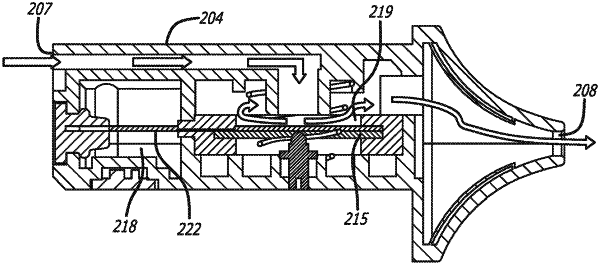
a mist generator housing which is elongate and comprises an air inlet port and a mist outlet port;
a liquid chamber configured to hold a liquid to be atomised;
a sonication chamber provided within the mist generator housing;
a capillary element extending between the liquid chamber and the sonication chamber such that a first portion of the capillary element is within the liquid chamber and a second portion of the capillary element is within the sonication chamber;
a divider which provides a barrier between the liquid chamber and the sonication chamber, wherein the divider includes a capillary aperture through which part of the first portion of the capillary element extends
an ultrasonic transducer having a generally planar atomisation surface which is provided within the sonication chamber, the ultrasonic transducer being mounted within the mist generator housing such that the plane of the atomisation surface is substantially parallel with a longitudinal length of the mist generator housing, wherein part of the second portion of the capillary element is positioned over at least a part of the atomisation surface, and wherein the ultrasonic transducer is configured to vibrate the atomisation surface to atomise the liquid carried by the second portion of the capillary element to generate the mist comprising the atomised liquid and air within the sonication chamber; and
an airflow channel which provides an air flow path between the air inlet port, the sonication chamber and the air outlet port such that a user drawing on the mist outlet port draws air through the inlet port, through the sonication chamber and out through the mist outlet port, with the mist generated in the sonication chamber being carried by the air out through the mist outlet port for inhalation by the user;
a driver device comprising:
a battery;
an AC driver for converting a voltage from the battery into an AC drive signal at a predetermined frequency to drive the ultrasonic transducer;
an active power monitor for monitoring the active power used by the ultrasonic transducer when the ultrasonic transducer is driven by the AC drive signal, wherein the active power monitor provides a monitoring signal which is indicative of the active power used by the ultrasonic transducer;
a processor for controlling the AC driver and for receiving the monitoring signal drive from the active power monitor; and
a memory storing instructions which, when executed by the processor, cause the processor to:
A) control the AC driver to output an AC drive signal to the ultrasonic transducer at a predetermined sweep frequency;
B) calculate the active power being used by the ultrasonic transducer based on the monitoring signal;
C) control the AC driver to modulate the AC drive signal to maximise the active power being used by the ultrasonic transducer;
D) store a record in the memory of the maximum active power used by the ultrasonic transducer and the sweep frequency of the AC drive signal;
E) repeat steps A-D for a predetermined number of iterations with the sweep frequency incrementing with each iteration such that, after the predetermined number of iterations has occurred, the sweep frequency has been incremented from a start sweep frequency to an end sweep frequency;
F) identify from the records stored in the memory an optimum frequency for the AC drive signal which is the sweep frequency of the AC drive signal at which a maximum active power is used by the ultrasonic transducer; and
G) control the AC driver to output an AC drive signal to the ultrasonic transducer at the optimum frequency to drive the ultrasonic transducer to atomise the liquid.
|