| CPC A61K 31/4192 (2013.01) [A61P 35/00 (2018.01)] | 17 Claims |
|
1. A method of treating a cancer selected from chemotherapy-resistant acute myeloid leukemia, cytarabine-resistant acute myeloid leukemia, acute monocytic leukemia, diffuse mixed cell lymphoma, myelodysplastic syndrome, primary effusion lymphoma, erythroleukemia, chronic myeloid leukemia, chronic monocytic leukemia, double hit diffuse large B cell lymphoma, triple hit diffuse large B cell lymphoma, biliary tract cancer or cancer of the ampulla of Vater, non-small cell lung cancer, bronchoalveolar carcinoma, liver cancer, cancer of the ovary, and cancer of the upper aerodigestive tract in a subject, comprising administering to the subject a therapeutically effective amount of a compound represented by the following structural formula:
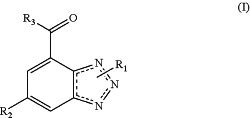 or a pharmaceutically acceptable salt thereof; wherein:
the dotted lines [ . . . ] in the ring represent an optional bond which may be present in any stable combination;
R1 is selected from hydrogen and alkyl;
R2 is -A-R4;
A is arylene or tetrasubstituted arylene; wherein the substituent is halogen;
R3 is selected from hydroxy and amino;
R4 is selected from an aryl and a heteroaryl that is optionally substituted with one or more R5;
R5 is selected from alkyl and —(CH2)nN(Ra)Rb;
Ra and Rb are independently selected from hydrogen, alkyl and —C(O)alkyl;
alternatively Ra and Rb can be taken together with the nitrogen atom to which they are attached to form a 4-6 membered heterocyclyl containing 0-2 additional heteroatoms independently selected from O and N and is optionally substituted with alkyl; and
n is an integer selected from 0 and 1.
|